Table 10.
Catalytic systems, major products, maximum FEs, and mechanisms of Pd and Pt complexes in electrochemical CO2 reduction (n.a.=not available, prop.=proposal, comp.=computational investigation, exp.=experimental evidence).
|
Entry |
Cat. system |
Substitution |
Major product |
Max. FE (%) |
Mechanism |
Basis |
Method |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
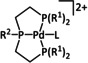
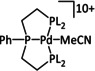
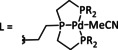
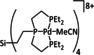
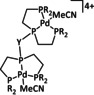
1a |
|
L=MeCN, PEt3, PPh3, P(CH2OH)3, P(OMe)3 R1=Et, neopentyl, Cy, Ph, (CH2)2PO(OEt)2, NMe2 R2=tBu, neopentyl, Ph, Mes, (CH2)3OH, TMB,[a] (CH2)2PMe3 +, (CH2)2PBu3 +, NMe2, NEt2, NiPr2, OMe |
CO/H2O CH4 H2 |
94 [319] 11 [319] 90 [316] |
ETH [320] ETM [316] |
prop. exp. |
n.a. CV |
[316, 319–321] |
|
|
|
|
|
|
|
|
|
|
|
1b |
|
R=Et, Ph |
CO/H2O H2 |
25 87 |
n.a. |
n.a. |
n.a. |
|
|
1c |
|
– |
CO/H2O H2 |
37 68 |
n.a. |
n.a. |
n.a. |
|
|
1d |
|
R=Et, Cy Y=CH2, 1,3‐phenyl |
CO/H2O H2 |
85 [323] 26 [324] |
ETM |
exp. |
CV |
|
|
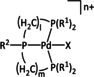
1e |
|
l=2, 3; m=1, 2, 3; n=1, 2 R1=Et, Cy; R2=Ph, Mes X=H, MeCN |
CO/H2O H2 |
97 39 |
ETM |
prop. |
n.a. |
|
|
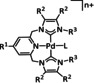
2 |
|
L=MeCN, Cl, Br; n=1, 2 R1=H, Br, OMe, CO2(CH2)4OH; R2=H R2∩R2=Ph, pyr, phenanthrene R3=Bu, CH2PyMe+, propylNMe3 +, propylPEt3 +, propylPPh3 +, CH2C6H4PPh3 + |
CO H2 |
52 [326] 100 [326] |
ETM |
comp. |
DFT |
[305, 317, 326, 327] |
|
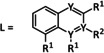
3 |
[PdL2Cl2] |
L=pyrazole, 4‐methylpyridine, 3‐methylpyrazole |
HCO2 − H2 |
20 54 |
ETM |
prop. |
n.a. |
|
|
|
|
|
|
|
|
|
|
|
|
4a |
[Pd(PPh3)2L]+ |
R1= –, H, OH; R2= –, Me, OH; Y=C, N |
CO/H2O HCO2 − |
75 45 |
ETM |
prop. |
n.a. |
|
|
|
|
|
|
|
|
|
|
|
|
4b |
[Pd(PPh3)2L]2+ |
L=4,4′‐dimethyl‐2,2′‐bpy, 4‐methyl‐1,10‐phen |
CO/H2O HCO2 − |
81 40 |
ETM |
prop. |
n.a. |
|
|
|
|
|
|
|
|
|
|
|
|
5 |
[Pt(dmpe)2]2+ |
dmpe=1,2‐bis(dimethyl‐ phosphino)ethane |
HCO2 − |
90 [318] |
ETH |
exp. [329] |
CV, NMR |
[a] TMB=trimethoxybenzyl.