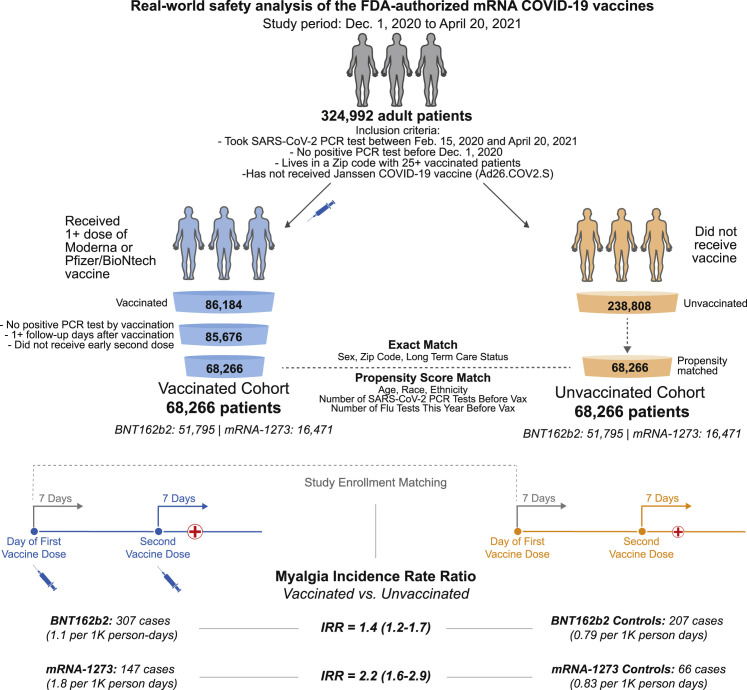
Figure 1.
Schematic illustration of participant selection and study design
The vaccinated cohort is composed 68,266 individuals from the Mayo Clinic and associated health systems who received at least one dose of BNT162b2 (Pfizer/BioNTech; n = 51,795) or mRNA-1273 (Moderna; n = 16,471) between December 1, 2020 and April 20, 2021 and did not test positive for SARS-CoV-2 prior to their first vaccination. A control cohort of unvaccinated individuals was generated via a combination of exact matching parameters and one-to-one propensity score matching, yielding 68,266 individuals with similar distributions of age, sex, race, ethnicity, residential location, number of prior influenza and SARS-CoV-2 PCR tests in the past year, and current long-term care residence status. For each cohort, the incidence rates of several adverse effects (e.g., myalgia) were calculated for the 7 days following the first dose and, separately, for the 7 days following the second dose. For a given adverse effect, the incidence rate ratio (IRR) and the corresponding 95% confidence interval (CI) were calculated to determine whether individuals receiving BNT162b2 or mRNA-1273 were more likely to experience the event during these intervals than their matched unvaccinated controls. Incidence rates and IRRs were also calculated for the 14 and 21 days following each vaccine dose.