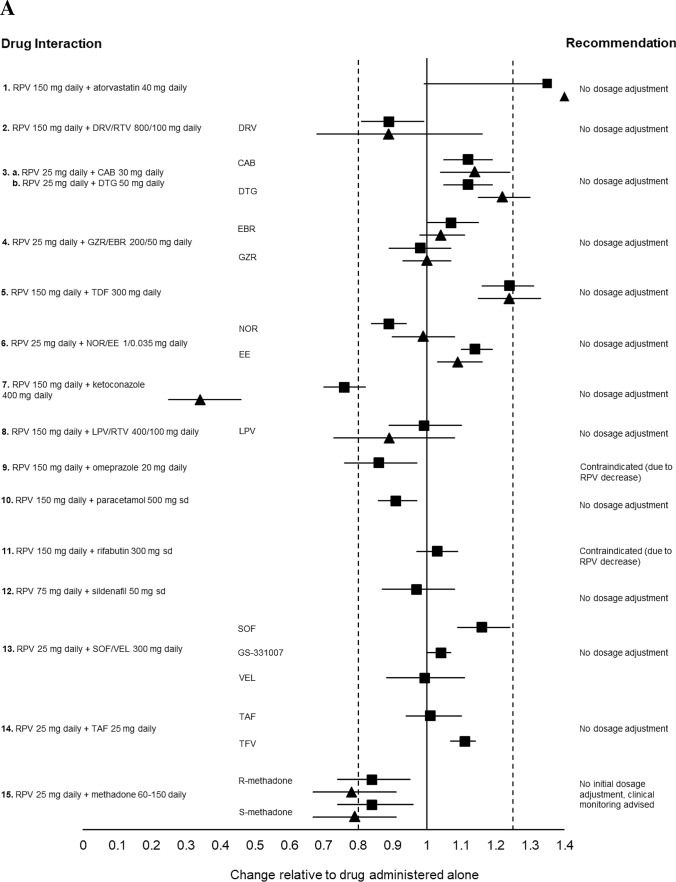
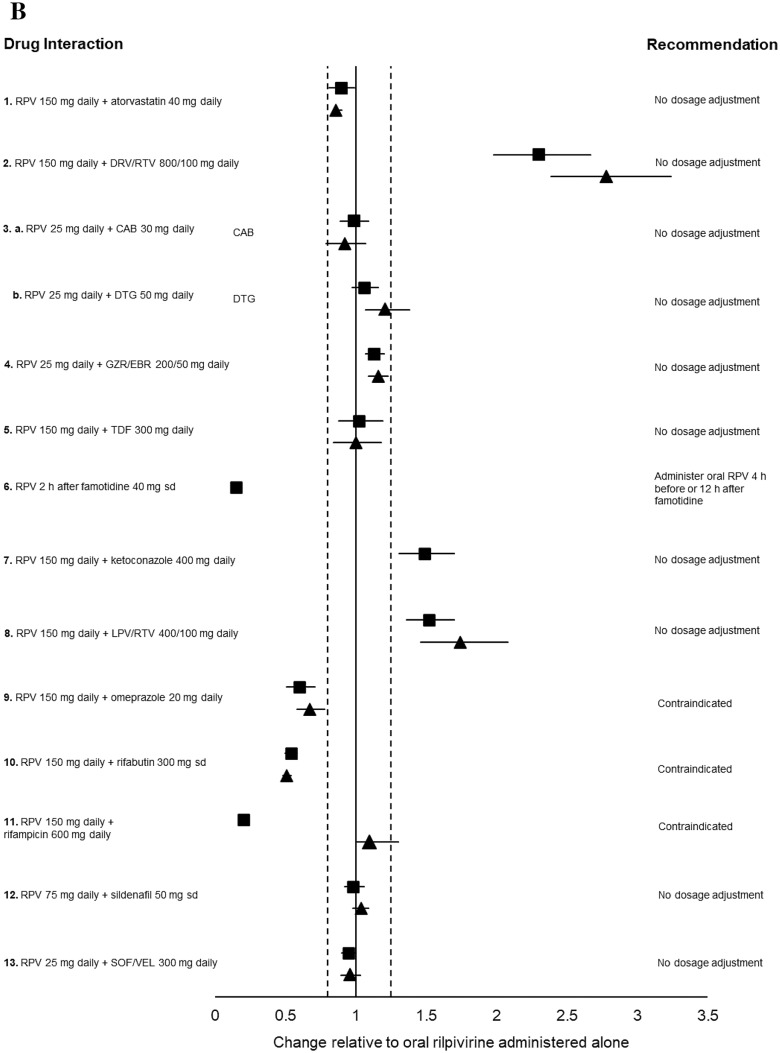
Fig. 3.
a Impact of oral rilpivirine on co-medication pharmacokinetics from drug–drug interaction crossover studies in healthy volunteers. 1. Period 1: Atorvastatin 40 mg daily for 4 days. Period 2: Rilpivirine 150 mg daily for 15 days + atorvastatin 40 mg daily days 12–15 (n = 16). 2. Period 1: Rilpivirine 150 mg daily for 11 days. Period 2: Darunavir/ritonavir 800/100 mg daily for 22 days + rilpivirine 150 mg daily day 12–22 (n = 16). 3a. Period 1: Cabotegravir 30 mg daily for 12 days. Period 2: Rilpivirine 25 mg daily for 11 or 12 days. Period 3: Cabotegravir 30 mg daily + rilpivirine 25 mg daily, for 12 days (n = 11). 3b. Period 1: Dolutegravir 50 mg daily for 5 days. Period 2: Rilpivirine 25 mg daily for 11 or 12 days. Period 3: Dolutegravir 50 daily + rilpivirine 25 mg daily for 5 days (n = 16). 4. Period 1: Grazoprevir/elbasvir 200/50 mg daily for 8 days. Period 2: Rilpivirine 25 mg daily for 11 days. Period 3: Grazoprevir/elbasvir 200/50 mg daily + rilpivirine 25 mg daily for 9 days (n = 20). 5. Period 1: Rilpivirine 150 mg daily for 8 days. Period 2: Tenofovir disoproxil fumarate 300 mg daily for 16 days + rilpivirine 150 mg daily either days 1–8 or days 9–16 (n = 15). 6. Period 1: Norethindrone/ethinylestradiol 1/0.035 mg in three 21-day cycles + rilpivirine 25 mg daily on days 1–15 of the third cycle (n = 18). 7. Period 1: Rilpivirine 150 mg daily for 11 days. Period 2: Ketoconazole 400 mg daily for 22 days + rilpivirine 150 mg daily days 12–22. 8. Period 1: Rilpivirine 150 mg daily for 10 days. Period 2: Lopinavir/ritonavir 400/100 mg twice daily for 20 days + rilpivirine 150 mg days 11–20 (n = 14). 9. Period 1: Rilpivirine 150 mg daily for 11 days. Period 2: Omeprazole 20 mg daily for 22 days + rilpivirine 150 mg on days 12–22 (n = 16). 10. Period 1: Paracetamol 500 mg, single dose. Period 2: Rilpivirine 150 mg daily for 12 days + paracetamol 500 mg single dose on day 11 (n = 16). 11. Period 1: Rilpivirine 150 mg daily for 11 days. Period 2: Rifabutin 300 mg daily for 11 days. Period 3: Rilpivirine 150 mg + rifabutin 300 mg daily for 11 days (n = 18). 12. Period 1: Sildenafil 50 mg, single dose. Period 2: Rilpivirine 75 mg daily for 12 days + sildenafil 50 mg single dose on day 12 (n = 16). 13. Period 1: Sofosbuvir/velpatasvir 400/100 mg daily for 8 days. Period 2: Emtricitabine/rilpivirine/tenofovir disoproxil fumarate 200/25/300 mg + sofosbuvir/velpatasvir 400/100 mg daily for 8 days (n = 24). 14. Period 1: Rilpivirine 25 mg daily for 14 days. Period 2: Tenofovir alafenamide fumarate 25 mg daily for 14 days. Period 3: Rilpivirine 25 mg + tenofovir alafenamide fumarate 25 mg daily, for 14 days (n = 17). 15. Stable methadone dose (60–150 mg daily) for 25 days + rilpivirine 25 mg daily on days 15–25 (n = 13). (b) Impact of co-medications on oral rilpivirine pharmacokinetics from drug–drug interaction crossover studies in healthy volunteers. 1. Period 1: Atorvastatin 40 mg daily for 4 days. Period 2: Rilpivirine 150 mg daily for 15 days + atorvastatin 40 mg daily days 12–15 (n = 16). 2. Period 1: Rilpivirine 150 mg daily for 11 days. Period 2: Darunavir/ritonavir 800/100 mg daily for 22 days + rilpivirine 150 mg daily days 12–22 (n = 16). 3a. Period 1: Cabotegravir 30 mg daily for 12 days. Period 2: Rilpivirine 25 mg daily for 11 or 12 days. Period 3: Cabotegravir 30 mg daily + rilpivirine 25 mg daily for 12 days (n = 11). 3b. Period 1: Dolutegravir 50 mg daily for 5 days. Period 2: Rilpivirine 25 mg daily for 11 or 12 days. Period 3: Dolutegravir 50 daily + rilpivirine 25 mg daily for 5 days (n = 16). 4. Period 1: Grazoprevir/elbasvir 200/50 mg daily for 8 days. Period 2: Rilpivirine 25 mg daily for 11 days. Period 3: Grazoprevir/elbasvir 200/50 mg daily + rilpivirine 25 mg daily for 9 days (n = 20). 5. Period 1: Rilpivirine 150 mg daily for 8 days. Period 2: Tenofovir disoproxil fumarate 300 mg daily for 16 days + rilpivirine 150 mg daily either days 1–8 or days 9–16 (n = 15). 6. Period 1: Rilpivirine 150 mg single dose. Period 2: Rilpivirine 150 mg single dose 2 h after famotidine 40 mg single dose (n = 24). 7. Period 1: Rilpivirine 150 mg daily. Period 2: Ketoconazole 400 mg daily for 22 days + rilpivirine 150 mg daily days 12–22 (n = 14). 8. Period 1: Rilpivirine 150 mg daily for 10 days. Period 2: Lopinavir/ritonavir 400/100 mg twice daily for 20 days + rilpivirine 150 mg days 11–20 (n = 14). 9. Period 1: Rilpivirine 150 mg daily for 11 days. Period 2: Omeprazole 20 mg daily for 22 days + rilpivirine 150 mg on days 12–22 (n = 16). 10. Period 1: Rilpivirine 150 mg daily for 11 days. Period 2: Rifabutin 300 mg daily for 11 days. Period 3: Rilpivirine 150 mg + rifabutin 300 mg daily for 11 days (n = 18). 11. Period 1: Rilpivirine 150 mg daily. Period 2: Rilpivirine 150 mg daily + rifampicin 600 mg daily (n = 16). 12. Period 1: Sildenafil 50 mg, single dose. Period 2: rilpivirine 75 mg daily for 12 days + sildenafil 50 mg single dose on day 12 (n = 16). 13. Period 1: Sofosbuvir/velpatasvir 400/100 mg daily for 8 days. Period 2: Emtricitabine/rilpivirine/tenofovir disoproxil fumarate 200/25/300 mg + sofosbuvir/velpatasvir 400/100 mg daily for 8 days (n = 24). Data are presented as area under the curve (squares) and trough plasma concentration (triangles) geometric mean ratios + 90% confidence intervals for rilpivirine with and without co-medication. The bioequivalence margin (0.8–1.25) is indicated by dashed vertical lines. CAB cabotegravir, DRV/RTV darunavir/ritonavir, DTG dolutegravir, GS-331007 metabolite of sofosbuvir, GZR/EBR grazoprevir/elbasvir, LPV/RTV lopinavir/ritonavir, NOR/EE norgestrel/ethinylestradiol, RPV rilpivirine, sd single dose, SOF/VEL sofosbuvir/velpatasvir, TAF tenofovir alafenamide, TDF tenofovir disoproxil fumarate [49, 71–83, 88, 95, 96].