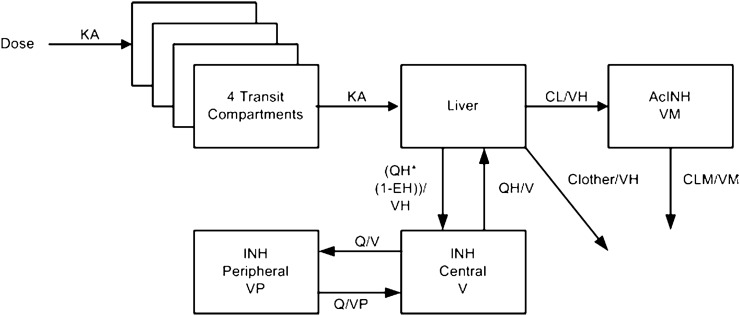
Fig. 1.
Schematic overview of the final isoniazid (INH) population pharmacokinetic model. The dose enters the well-stirred liver compartment via a four-compartmental transit model. Absorption constant (KA) is defined as the number of transit compartments plus 1, divided by the mean transit time (MTT). From the liver compartment, the drug can be distributed to the central INH compartment or be metabolized into acetyl-isoniazid (AcINH) or other metabolites. From the central AcINH compartment, the drug is cleared through first-order elimination. Isoniazid pharmacokinetics are described using a two-compartment disposition model and AcINH pharmacokinetic by a one-compartment disposition model. CL clearance into acetyl-isoniazid, CL other clearance into other metabolites than acetyl-isoniazid, CLM clearance of the acetyl-isoniazid metabolite, EH hepatic extraction ratio, Q inter-compartmental clearance, QH hepatic plasma flow, V central volume of isoniazid, VH hepatic volume, VM central volume of the acetyl-isoniazid metabolite, VP peripheral volume of isoniazid