Figure 2. Pancreatic cancers rely on autophagy and lysosomal scavenging.
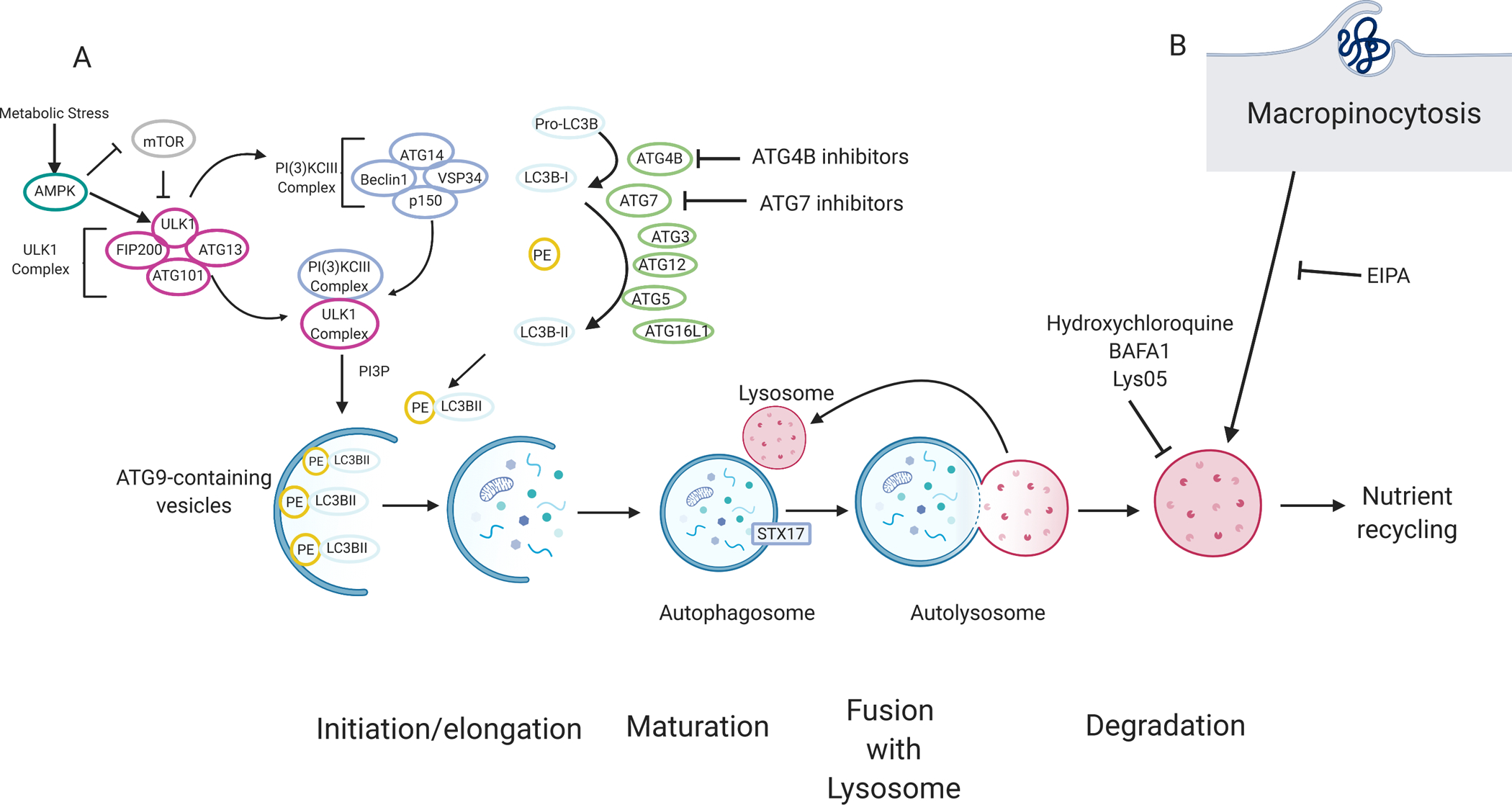
(A)70The molecular mechanism of autophagy can be categorized by four main stages: initiation and/or elongation, closure, fusion with the lysosome, and degradation. Nutrient starvation is a major trigger for autophagy activation through mTOR and AMPK. A series of protein–lipid interactions convert pro-LC3B into LC3B-I by ATG4B. LC3B is subsequently conjugated into phosphatidylethanolamine (PE), producing LC3B-II, via ubiquitin conjugation-like E1-E2-E3 series enzymes ATG7, ATG3, and ATG12-ATG5-ATG16L1 respectively. LC3B-II is associated with the autophagosome and acts as a docking site for autophagy cargo receptors. Cargo, such as damaged proteins or organelles are sequestered in a double membrane vesicle called an autophagosome. Ultimately, the autophagosome fuses with the lysosome, and the content is degraded. Degraded products are shuttled back into the cytoplasm where they can be used in various metabolic pathways. Autophagy and lysosomal degradation can be inhibited by compounds such as hydroxychloroquine, Lys05, and bafilomycin (BAFA1). Other compound tools targeting other modulators of autophagy such as ATG4B inhibitors are currently being tested. (B) Macropinocytosis, a non-selective endocytic pathway, allows extracellular content, such as albumin, to be degraded by the lysosome and can provide amino acids and other metabolites to the cell. Macropinocytosis can be inhibited by EIPA (5-(N-ethyl-N-isopropyl)-Amiloride).
