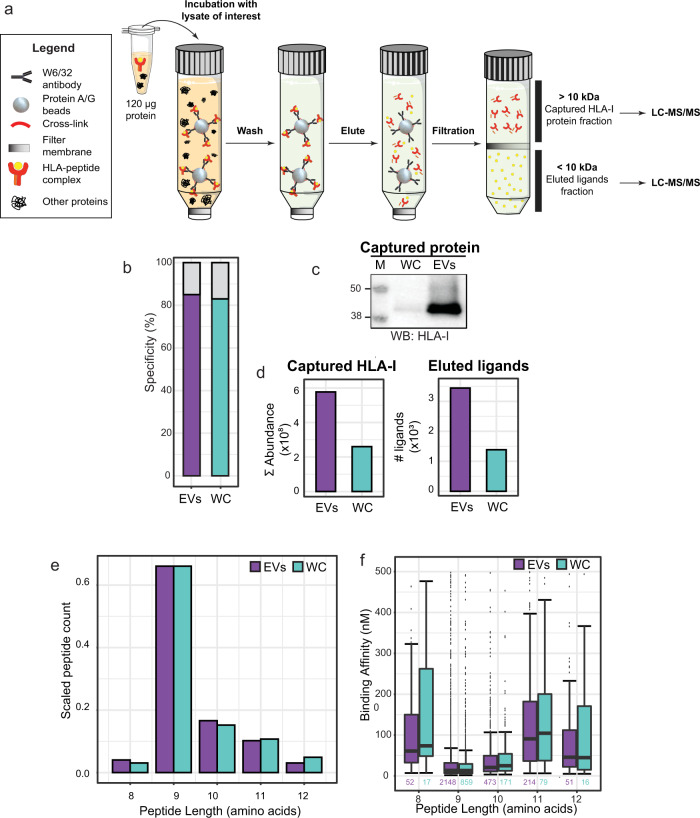
Fig. 2. Global characterization of the EV HLA-I peptide ligandome.
a Preparative steps in the down-scaled HLA-I complex retrieval workflow. An input load of 120 μg was used for immunoaffinity purification of HLA-I complexes from both WCL and EVL. The flowthrough from 10 kDa filtration contains HLA peptide ligands that were subjected to LC-MS/MS analysis. The retentate contains HLA-I and HLA-I interacting proteins, which were digested, for the analysis of HLA-I abundance and interactome. b HLA-I peptide ligand specificity. From the 10 kDa flowthrough fraction, more than 80% of peptides detected were predicted to bind to the JY HLA type (HLA-A*02:01; HLA-B*07:02; HLA-C*07:02). c Western blot (WB) detection of HLA-I. HLA-I was detected in the eluate of WCL HLA-I IP, and more strongly in the eluate of EVL HLA-I IP. Full image provided in Supplementary Fig. 9. d Mass spectrometry analysis of immuno-purified HLA-I proteins and HLA-I peptide ligands. From EVs, more HLA-I proteins (cumulative MS intensities of HLA-A, HLA-B and HLA-C) and HLA-I peptide ligand species were detected. e Peptide length distribution. HLA-I peptide ligands from both whole-cells (WC) and EVs distributed similarly, and expectedly, between 8 to 12 amino acids. f Predicted binding affinity. Marginal differences in predicted binding affinity were observed in HLA-I peptides retrieved from WC and EVs. Box plots represent n peptides (where n has been annotated under each box). The 25%, 50% (median), and 75% quantiles are represented in each box, and the whiskers represent the ±1.5*IQR from the closest quantile. Bar plots represent the total pool of eluted ligands detected in three technical replicates from either WC (green) or EVs (purple).