Abstract
The inability to over-express Aquaporin 6 (AQP6) in the plasma membrane of heterologous cells has hampered efforts to further characterize the function of this aquaglyceroporin membrane protein at atomic detail using crystallographic approaches. Using an Aquaporin 3-tGFP Reporter (AGR) system we have identified a region within loop C of AQP6 that is responsible for severely hampering plasma membrane expression. Serine substitution corroborated that amino acids present within AQP6194–213 of AQP6 loop C contribute to intracellular endoplasmic reticulum (ER) retention. This intracellular retention signal may preclude proper plasma membrane trafficking and severely curtail expression of AQP6 in heterologous expression systems.
Subject terms: Molecular biology, Structural biology
Introduction
Aquaporins are proteins composed of six transmembrane α-helices that form a single water-permeable channel. All monomeric pores exhibit a conserved Asn-Pro-Ala (NPA)1 constriction site formed by two short half helical segments and a selectivity filter (SF) at the narrowest point in the channel. All aquaporins are further composed of four monomers, held together by hydrophobic interactions which create a tetramer that displays a central pore2. Aquaporins are traditionally divided into two categories: strict water channels and aquaglyceroporins, which in addition to water also transport glycerol and other small solutes via the monomeric pore3–5. This classification has expanded considerably over the past decade as more evidence has emerged of potential gas permeability in aquaporins. Boron et al. first demonstrated that Aquaporin 1 (AQP1) expressed in oocytes is also permeable to CO26. Molecular dynamic simulations7 and monomeric pore inhibitor studies8 suggest that approximately half the channel-dependent flux of CO2 moves through the four independent water pores, whereas the other half might permeate via the central pore pathway.
Aquaporin 6 is localized mainly in the kidney and it co-localizes with the vH + -ATPase membrane protein. Interestingly, although AQP6 is technically classified as an aquaglycerolporin, its highest sequence homology is more closely related to pure water channels such as AQP0, AQP2 and AQP5. Aquaporin 6 has an additional unique property of being permeable to anions under low (acidic) pH levels9. It is therefore surmised that AQP6 may be involved in acid–base regulation10. For example, a single amino acid substitution of asparagine by glycine at position 60 (N60G) eliminates AQP6 anion permeability completely when expressed in Xenopus oocytes11. Interestingly, this occurs with a concomitant increase of water permeability, similar to AQP2, which is not inhibited by HgCl2. Oocytes studies performed by Boron et al.8,12 have shown that AQP6 is additionally permeable to NH3 as well. Similar N60G mutations in other aquaporins, such as AQP0, AQP1 and AQP2 result instead in a failure of the protein to traffic to the plasma membrane. This strongly suggests that the interaction of transmembrane domains 2 and 5 in AQP6 may result in significant conformational changes11 and perturbation of the native protein structure.
Currently, the inability to over-express AQP6 in heterologous systems is a major bottleneck precluding crystallographic studies. AQP6 basal expression in its native location of the kidney is already very low13, which makes purification from this source tissue not practicable. Transient transfection of AQP6 in insect or mammalian cell lines has not been successful either14.
Like most eukaryotic membrane proteins AQP6 is glycosylated. One potential glycosylation site, N134, is in the region of loop B and may be essential for translocation and function15. Therefore, heterologous expression of AQP6 would ideally be carried out in a system which has the ability for post-translational modifications.
In the present work we took advantage of a previously created Aquaporin 3-tGFP (AQP3-tGFP) construct16 that displays intense Plasma Membrane (PM) with low cytoplasmic fluorescence, termed the AQP3-tGFP-based Reporter (AGR) system. In the past, we have used this system to identify a novel endoplasmic reticulum (ER)-retention sequence located in the N-terminus of TMC117. We designated these ER-retention sequences “Omega-type” since their presence in a protein leads to a complete termination of PM localization17.
Using the AGR system we now have identified a region within the third extracellular loop of AQP6 that decreased PM localization when expressed in HEK293 cells. Using serine-mutagenesis we restored the ability of this region to reach the PM using the AGR system. We hypothesize that the third extracellular loop of AQP6 with the sequence FTGCSMNPARSFGPAVIVGKFAVHWIF harbors an uncharacterized ER retention sequence that may explain the inability to over-express AQP6 in heterologous cell systems.
Results
Expression and localization of tagged AQP6 in HEK293 cells
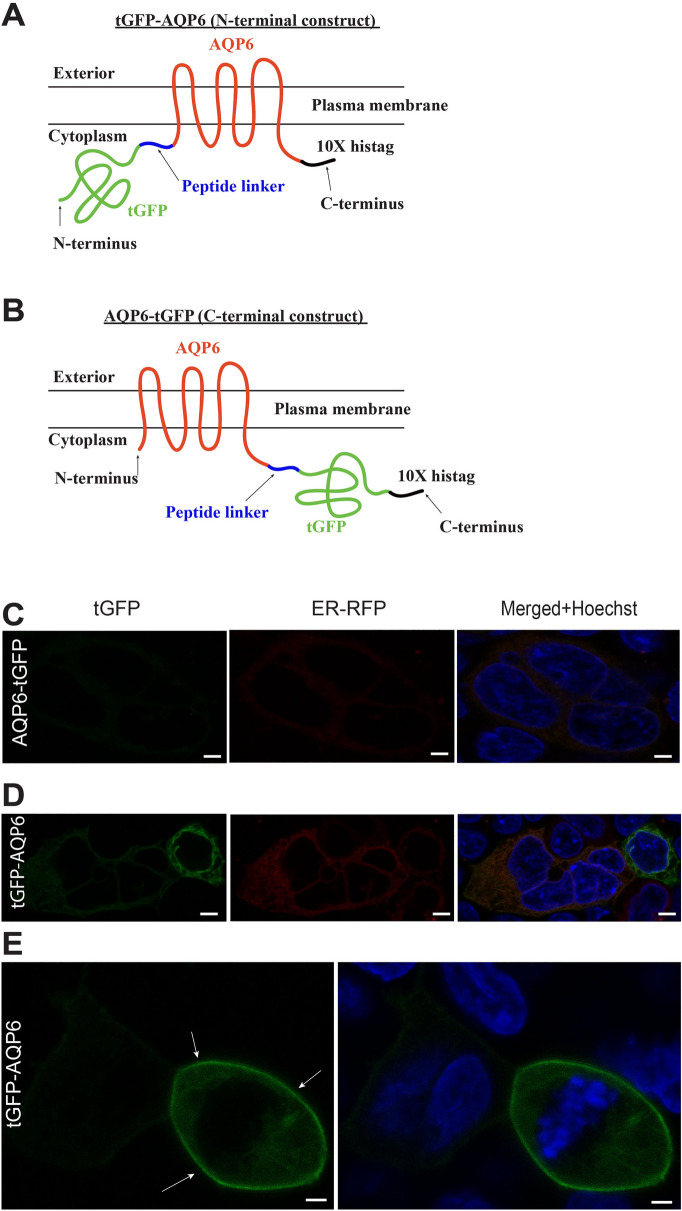
To study the cellular localization of AQP6 in transiently transfected HEK293 cells, two tagged constructs were made using turbo GFP (tGFP) at the N- or C-terminus of AQP6 (Fig. 1A,B respectively). The C-terminal tGFP tagged construct produced almost no AQP6 expression as previously reported by Ikeda et al.18 with a complete absence of plasma membrane (PM) localization (Fig. 1C). Although the N-terminal tGFP tagged version displayed higher levels of expression, localized mainly in the ER (Fig. 1D). However, we were able to identify a small number of transfected cells displaying PM localization (Fig. 1E) confirming previous reports using a different heterologous system19. However, in both N- and C-terminal versions, expression levels of AQP6 were so low overall that any attempt to use these constructs for mass-production of AQP6 for structural studies is unlikely.
Figure 1.
AQP6 expressed in transient heterologous transfection constructs rarely reaches the plasma membrane. (A) The N-terminal tGFP-labeled AQP6 construct is shown (tGFP-AQP6). The peptide linker is shown in blue and the C-terminal 10× Histag in black. (B) The C-terminal GFP-labeled AQP6 construct is shown (AQP6-tGFP). The peptide linker is shown in blue and the C-terminal 10× Histag in black. (C) Transient transfection of an AQP6-tGFP construct in HEK293 cells yields very poor to no expression. (D) Transient transfection of tGFP-AQP6 construct in HEK293 cells improves expression but most of the expressed protein is retained in the ER. Scale bars (C, D): 5 µm. (E) Very rarely, the tGFP-AQP6 construct localizes to the PM (white arrows) in HEK293 cells. Scale bar: 3 µm. Cells were observed microscopically using an upright Olympus BX51WI equipped with 100 × 1 NA objective, and images were captured using a Grasshopper3 CMOS camera (FLIR, Richmond, BC, Canada) controlled by Leica LAS X version 3.5 software (available at: https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/). All figures were created using Adobe Illustrator CS6 (available at: https://adobe.com/products/illustrator), under an Adobe Inc., Creative Cloud Desktop 2019 shared device license to Case Western Reserve University (CWRU) that operates until 3/31/2022.
Scanning AQP6 residues 1–138 using the AGR system
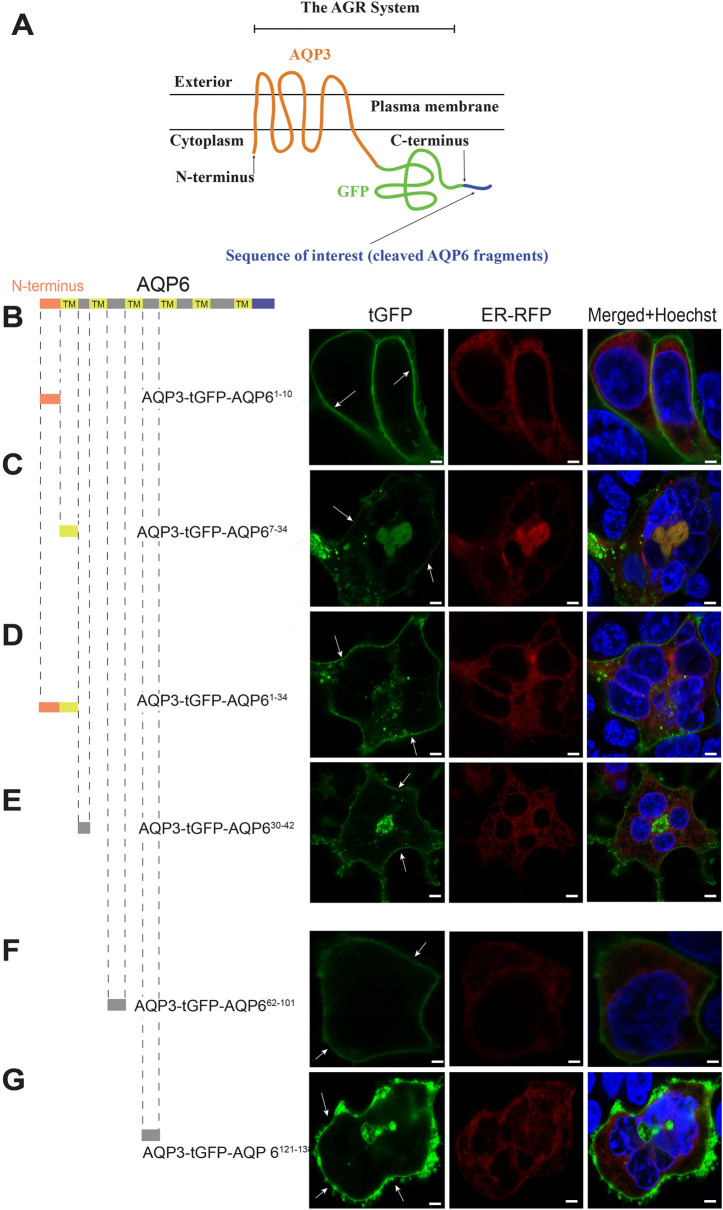
In order to identify potential regions of AQP6 that may be responsible for reducing expression levels (thus containing uncharacterized ER retention or degron motifs20) we used our previously described AGR system17 (Fig. 2A). Because the AGR system displays very strong PM localization with minimal cytoplasmic staining, any given protein can be cleaved into peptide fragments, and each peptide fragment independently C-terminally attached to AGR to check for its cellular localization potential. If a particular amino acid peptide domain attached to AGR leads to decreased PM localization, it is inferred that this domain may contain an ER-retention motif. If instead PM localization occurs, we then conclude the peptide fragment does not express an Omega-type ER-retention motif. Using the AGR system we tested the intracellular and extracellular loops of AQP6, obviating its transmembrane domains (TMD) except for the initial domain. To our surprise, as shown in Fig. 2, neither AQP6 N-terminal—AQP61–10 domain (Fig. 2B), nor domains AQP67–34 (Fig. 2C), AQP61–34 (Fig. 2D), AQP630–42 (Fig. 2E), AQP662–101 (Fig. 2F), or AQP6121–138 (Fig. 2G) precluded PM localization when individually C-terminally attached to the AGR system. However, it is possible our system masked N-terminal targeting signals previously reported for AQP618,19.
Figure 2.
AGR scanning of the first half of AQP6. (A) Schematic representation for usage of the AGR system with AQP6 (Figure was adapted from Ref.17) under the terms of the Creative Commons CC BY license. (B) Attaching the N-terminus of AQP6 (comprising residues AQP61–10) C-terminally to AGR, results in a construct that reaches the PM (white arrows). (C) In a similar manner, when the first transmembrane domain (TMD) of AQP6 (comprising residues AQP67–34) is C-terminally attached to AGR, this construct also reaches the PM. (D) Using the entire AQP61–34 residues (which include N-terminus and first TMD), instead of the subunits indicated in (A, B), also results in PM expression when attached to AGR. (E) When residues AQP630–42 are used, this construct also localized to the PM. (F) Similarly, when peptide residues AQP662–101 are attached to AGR, the construct localized to the PM. Finally, (G) when AQP6121–138 peptide residues are used the construct also reached the PM. Cells were observed microscopically using an upright Olympus BX51WI equipped with 100 × 1 NA objective and images were captured using a Grasshopper3 CMOS camera (FLIR, Richmond, BC, Canada) controlled by Leica LAS X version 3.5 software (available at: https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/). All figures were created using Adobe Illustrator CS6 (Available at: https://adobe.com/products/illustrator), under an Adobe Inc., Creative Cloud Desktop 2019 shared device license to Case Western Reserve University (CWRU) that operates until 3/31/2022. Scale bars: 8 µm.
The third outside loop of AQP6 precludes PM localization of AGR
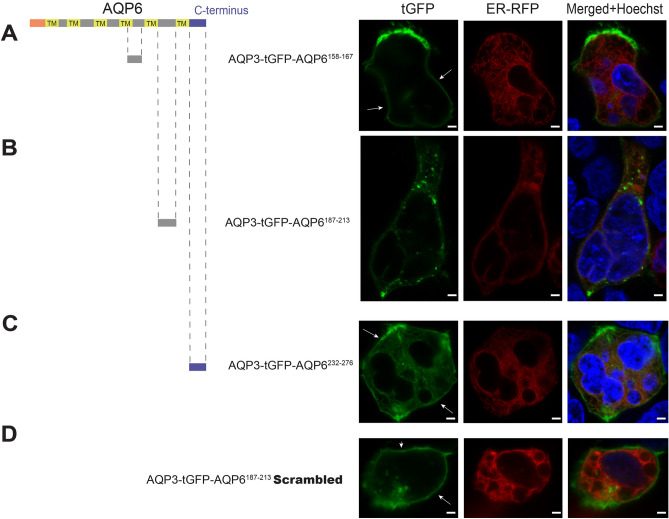
We then scanned the rest of the AQP6 loops, individually attaching peptide fragment domains from AQP6158–167 (Fig. 3A), AQP6187–213 (Fig. 3B) and the C-terminus AQP6232–276 domain (Fig. 3C) to the C-terminal of the AGR system. Interestingly, the peptide domain AQP6187–213 produced a marked decrease in expression and a total absence of PM localization of the AGR reporter (Fig. 3B). In contrast, a scrambled version of AQP6187–213 was able to reach the PM when C-terminally attached to AGR (Fig. 3D), suggesting the presence of an uncharacterized sequence-specific ER retention motif within region AQP6187–213.
Figure 3.
AGR scanning of the second half of AQP6. (A) Approaching the second half of AQP6 in a similar manner to Fig. 2, peptide residues AQP6158–167 when C-terminally attached to AGR results in a construct that reaches the PM (white arrows). (B) However, when residues AQP6187–213 are C-terminally attached to AGR, the construct does not reach the PM. (C) Although, AQP6232–276 residues (compromising AQP6 C-terminal) attached to AGR does localize to the PM. (D) Interestingly, when a scrambled version of peptide residues AQP6187–213 are substituted for the native AQP187–213 and C-terminally attached to AGR, this construct is able to reach the PM. Cells were observed microscopically using an upright Olympus BX51WI equipped with 100 × 1 NA objective and images were captured using a Grasshopper3 CMOS camera (FLIR, Richmond, BC, Canada) controlled by Leica LAS X version 3.5 software (available at: https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/). All figures were created using Adobe Illustrator CS6 (Available at: https://adobe.com/products/illustrator), under an Adobe Inc., Creative Cloud Desktop 2019 shared device license to Case Western Reserve University (CWRU) that operates until 3/31/2022. Scale bars: 8 µm.
Mutagenesis of loop C identifies the retention region
In order to further pinpoint the region that produces the PM targeting in the AGR system, as shown in Fig. 3B, we undertook a systemic serine-mutagenesis approach in order to further elucidate the characteristics of “loop C” (Fig. 4A). Using four different constructs, each C-terminally attached to AGR, we identified the amino acid sequence PARSFGPAVIVGKFAVHWIF as the one responsible for ER retention (Fig. 4B–E).
Figure 4.
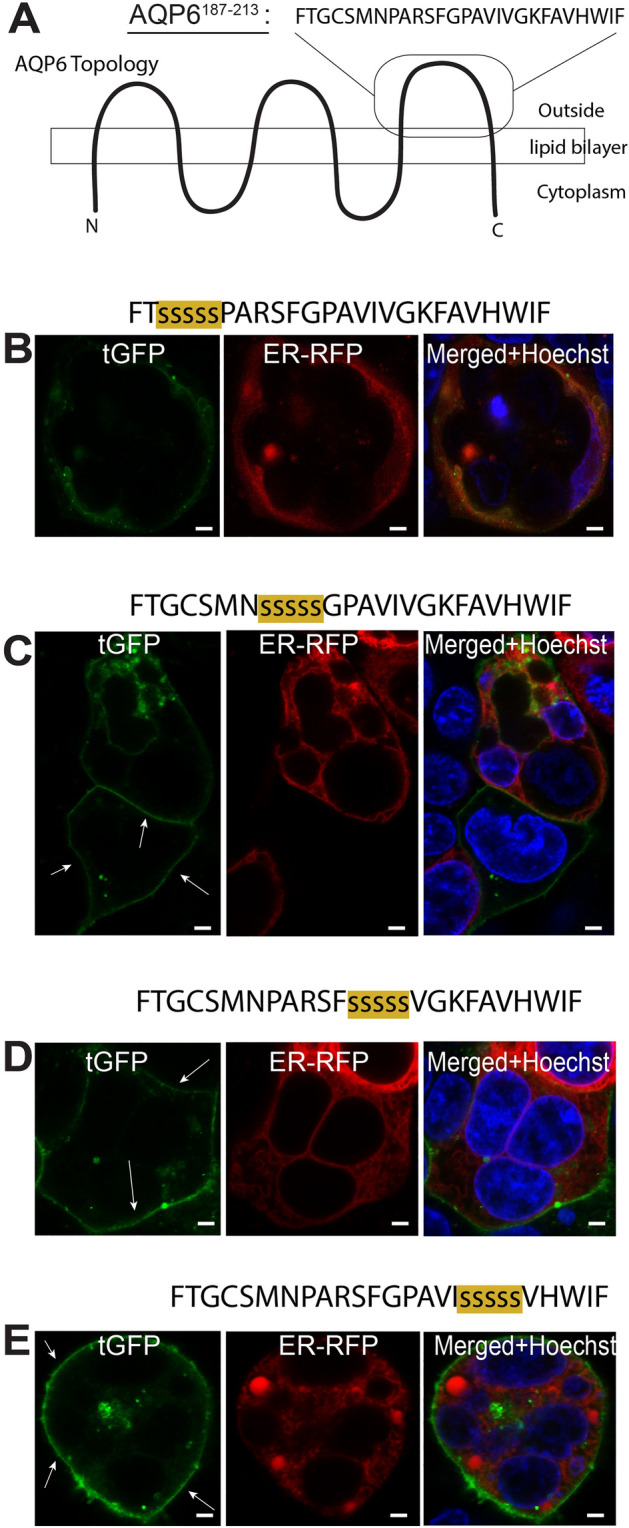
Serine mutagenesis pinpoints Omega signal localization. (A) The amino acid sequence comprising peptide fragment AQP6187–213 is shown. (B) When this peptide fragment was substituted with 5 serine substitutions and C-terminally attached to AGR, this construct fails to localize to the PM of HEK293 cells. In contrast, (C–E) amino acid serine substitutions within the indicated peptide fragments and then C-terminally attached to AGR, these constructs localize in the PM of HEK293 cells (white arrows). Cells were observed microscopically using an upright Olympus BX51WI equipped with 100 × 1 NA objective and images were captured using a Grasshopper3 CMOS camera (FLIR, Richmond, BC, Canada) controlled by Leica LAS X version 3.5 software (available at: https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/). All figures were created using Adobe Illustrator CS6 (Available at: https://adobe.com/products/illustrator), under an Adobe Inc., Creative Cloud Desktop 2019 shared device license to Case Western Reserve University (CWRU) that operates until 3/31/2022. Scale bars: 8 µm.
Discussion
Polytopic multi-pass membrane proteins are a notoriously difficult to mass express for functional and structural studies21. The aquaglyceroporin subtype AQP6 has been known for quite some time to be very difficult to express in heterologous systems18. In the present work, we sought to examine the molecular structure of AQP6, focusing on the cellular expression and localization characteristics of intracellular and extracellular AQP6 loops. Using the AGR system17, we were able to identify a region contained within the third extracellular loop of AQP6, so called “loop C”, that completely abrogated PM localization, while decreasing the expression levels of the AGR reporter system in HEK293 cells (Fig. 3B). To pinpoint whether or not this ER retention region could be abolished, we used serine-mutagenesis to localize the amino acid sequence containing the ER retention signal. As shown in Fig. 4, five consecutive amino acid substitutions along the underlined sequence FTGCSMNPARSFGPAVIVGKFAVHWIF restored PM localization in the AGR system (Fig. 4C,D), suggesting the retention motif is encoded within the underlined residues. Future studies will address the functionality of this sequence in the native AQP6 protein.
This novel insight may help in further designing a mutagenized AQP6 that displays high-expression levels when transiently expressed in heterologous cells such as HEK293. The ability to overcome the AQP6 ER-retention would improve the chances of producing sufficient quantities of AQP6 to allow crystallographic structural analysis. The AGR system has provided a potential explanation for why AQP6 expression levels remain very low even when heterologously expressed.
The current study does not address why such strong Omega-type ER-retention signals may be present in AQP6. One possible explanation is that proteins expressed in very specialized organs such as the kidneys for AQP6 or mechanosensing organelles such as the stereocilia for TMC1, depend on expression of tissue-specific chaperones that block Omega-type retention signals in order to enforce their tissue-specific effects. Thus attempts to express AQP6 or TMC1 proteins in non-native cell types leave their Omega-type signals exposed, and in the absence of tissue-specific chaperones expressed proteins default to ER-retention. Extended studies of Omega-type signals will attempt to address this hypothesis. Further work will also have to address the question of whether Omega-type ER retention signals can be abrogated using alternative amino acid substitution instead of alanines or serines in order to minimally disrupt the AQP6 native folding configuration.
Materials and methods
Plasmid constructs
The AQP3-tGFP-pcDNA5/FRT plasmid was synthesized as previously described17. The full cDNA containing human AQP6-tGFP (NP_001643) was synthesized by Biomatik (Wilmington, DE) and tGFP-AQP6 (rat NP_071517.1) by Genscript (Piscatawas, NJ). In the tGFP-AQP6 construct, the tGFP was separated from AQP6 by the following peptide linker which included a Precision Protease site (underlined in blue): AASAVNGSLEVLFQGPAA, and containing Afe1 and Hpa1 restriction sites, as well as a 10× C-terminal Histamine Tag (Histag) as shown in Fig. 1A (in black). For the AQP6-tGFP construct, the tGFP was separated from AQP6 by the following peptide linker (Fig. 1B in blue) which included a Precision Protease site: GGSLEVLFQGPAA and a c-terminal 10× Histag (in black) as shown in Fig. 1B.
The AQP6-tGFP construct was subcloned into a pcDNA5/FRT plasmid (Invitrogen, Carlsbad, CA) using restriction enzymes BamH1/EcoRV while the AQP6 was subcloned using Hpa1/EcoRI into a pcDNA5/FRT plasmid containing tGFP. Each construct was custom synthesized by Genscript, codon optimized for mammalian expression in HEK293 cells. Each AQP6-based sequence was then subcloned into the AQP3-tGFP-pcDNA5/FRT plasmid using Hpa1/EcoRV restriction sites. All constructs were confirmed by sequencing.
Cell culture, transfection
HEK293 cells were purchased from Life Sciences (Carlsbad CA) and seeded in 12-well glass-bottom culture plates (Cellvis, Mountain View, CA) and cultured in DMEM/F12 media (Life Technologies, Carlsbad, CA) supplemented with 10% Fetal Bovine Serum and 1% pen/strep for 3 days prior to transfection. Transfections were performed as described previously17. Briefly, on the day of transfection, spent media was replaced by fresh media and 500 ng of plasmid constructs were transfected into HEK293 cells using Lipofectamine 2000 (Life Technologies, Carlsbad, CA) following the manufacturer’s instructions. Red Fluorescent Protein based BacMam 2.0 constructs specific for ER were co-transfected the same day following the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). The cell nuclei was labelled using Hoechst dye (Life Technologies, CA). All recorded experiments were performed 48 h after transfection.
Imaging
Transfected HEK293 cells were examined with an SP8 confocal fluorescence microscope using 63 × 1.4 NA objective, Leica (Wetzlar, Germany) as described previously17. Briefly, cells were observed microscopically using an upright Olympus BX51WI equipped with 100 × 1 NA objective and images were captured using a Grasshopper3 CMOS camera (FLIR, Richmond, BC, Canada) controlled by Leica LAS X version 3.5 software (available at: https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/). All figures were created using Adobe Illustrator CS6 (Available at: https://adobe.com/products/illustrator), under an Adobe Inc., Creative Cloud Desktop 2019 shared device license to Case Western Reserve University (CWRU) that operates until 3/31/2022.
Acknowledgements
We would like to acknowledge use of the Leica TCS SP8 microscope in the Light Microscopy Imaging Facility at Case Western Reserve University, made available through the Office of Research Infrastructure (NIH-ORIP) Shared Instrumentation Grant S10OD024996. This research was partly supported by the Center for Clinical Research and Technology at University Hospitals Cleveland Medical Center (R.S.) and partly by NIH Grant DC015016 (R.S.) and ONR N000141612535. We would also like to thank Dr. David Lodowski for his assistance in properly citing copyright requirements.
Author contributions
D.C.S., T.K., T.S.M. and A.V.-F. wrote the manuscript and prepared all figures. A.E.S., K.D.C., R.S. and A.E. provided critical assistance in reviewing and editing the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vahedi-Faridi A, Engel A. Aquaporins in Health and Disease: New Molecular Targets for Drug Discovery. CRC Press; 2016. Aquaporin structure and selectivity; pp. 33–48. [Google Scholar]
- 2.Gonen T, Walz T. The structure of aquaporins. Q. Rev. Biophys. 2006;39(4):361–396. doi: 10.1017/S0033583506004458. [DOI] [PubMed] [Google Scholar]
- 3.Verkman AS. Mammalian aquaporins: Diverse physiological roles and potential clinical significance. Expert Rev. Mol. Med. 2008;10:e13. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu B, Beitz E. Aquaporins with selectivity for unconventional permeants. Cell Mol. Life Sci. 2007;64(18):2413–2421. doi: 10.1007/s00018-007-7163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heymann JB, Engel A. Aquaporins: Phylogeny, structure, and physiology of water channels. News Physiol. Sci. 1999;14:187–193. doi: 10.1152/physiologyonline.1999.14.5.187. [DOI] [PubMed] [Google Scholar]
- 6.Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am. J. Physiol. 1998;274(2):C543–548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Cohen J, Boron WF, Schulten K, Tajkhorshid E. Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J. Struct. Biol. 2007;157(3):534–544. doi: 10.1016/j.jsb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc. Natl. Acad. Sci. USA. 2009;106(13):5406–5411. doi: 10.1073/pnas.0813231106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aquaporin. Nature. 1999;402(6758):184–187. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- 10.Yasui M. pH regulated anion permeability of aquaporin-6. Handb. Exp. Pharmacol. 2009;190:299–308. doi: 10.1007/978-3-540-79885-9_15. [DOI] [PubMed] [Google Scholar]
- 11.Verkman AS. Aquaporins in clinical medicine. Annu. Rev. Med. 2012;63:303–316. doi: 10.1146/annurev-med-043010-193843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geyer RR, Musa-Aziz R, Qin X, Boron WF. Relative CO(2)/NH(3) selectivities of mammalian aquaporins 0–9. Am. J. Physiol. Cell Physiol. 2013;304(10):C985–994. doi: 10.1152/ajpcell.00033.2013. [DOI] [PubMed] [Google Scholar]
- 13.Yasui M, Kwon TH, Knepper MA, Nielsen S, Agre P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA. 1999;96(10):5808–5813. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eifler N, Duckely M, Sumanovski LT, et al. Functional expression of mammalian receptors and membrane channels in different cells. J. Struct. Biol. 2007;159(2):179–193. doi: 10.1016/j.jsb.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Krueger, A. Expression and purification of aquaporin-6 in different systems. Thesis dissertation (2012).
- 16.Soler DC, Young AE, Vahedi-Faridi A, McCormick TS. Generation of Flp-in(tm)-ready DG44 and Lec 3.2.8.1 CHO cell lines for quick and easy constitutive protein expression. Biotechniques. 2018;65(1):41–46. doi: 10.2144/btn-2018-0075. [DOI] [PubMed] [Google Scholar]
- 17.Soler DC, Manikandan M, Gopal SR, Sloan AE, McCormick TS, Stepanyan R. An uncharacterized region within the N-terminus of mouse TMC1 precludes trafficking to plasma membrane in a heterologous cell line. Sci. Rep. 2019;9(1):15263. doi: 10.1038/s41598-019-51336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasui M. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J. Biol. Chem. 2002;277(42):39873–39879. doi: 10.1074/jbc.M207008200. [DOI] [PubMed] [Google Scholar]
- 19.Beitz E, Liu K, Ikeda M, Guggino WB, Agre P, Yasui M. Determinants of AQP6 trafficking to intracellular sites versus the plasma membrane in transfected mammalian cells. Biol Cell. 2006;98(2):101–109. doi: 10.1042/BC20050025. [DOI] [PubMed] [Google Scholar]
- 20.Cho S, Dreyfuss G. A degron created by SMN2 exon 7 skipping is a principal contributor to spinal muscular atrophy severity. Genes Dev. 2010;24(5):438–442. doi: 10.1101/gad.1884910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter EP, Beis K, Cameron AD, Iwata S. Overcoming the challenges of membrane protein crystallography. Curr. Opin. Struct. Biol. 2008;18(5):581–586. doi: 10.1016/j.sbi.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]