Abstract
Depression associated with structural brain abnormalities is hypothesized to be related with accelerated brain aging. However, there is far from a unified conclusion because of clinical variations such as medication status, cumulative illness burden. To explore whether brain age is accelerated in never‐treated first‐episode patients with depression and its association with clinical characteristics, we constructed a prediction model where gray matter volumes measured by voxel‐based morphometry derived from T1‐weighted MRI scans were treated as features. The prediction model was first validated using healthy controls (HCs) in two Chinese Han datasets (Dataset 1, N = 130 for HCs and N = 195 for patients with depression; Dataset 2, N = 270 for HCs) separately or jointly, then the trained prediction model using HCs (N = 400) was applied to never‐treated first‐episode patients with depression (N = 195). The brain‐predicted age difference (brain‐PAD) scores defined as the difference between predicted brain age and chronological age, were calculated for all participants and compared between patients with age‐, gender‐, educational level‐matched HCs in Dataset 1. Overall, patients presented higher brain‐PAD scores suggesting patients with depression having an “older” brain than expected. More specially, this difference occurred at illness onset (illness duration <3 months) and following 2 years then disappeared as the illness further advanced (>2 years) in patients. This phenomenon was verified by another data‐driven method and significant correlation between brain‐PAD scores and illness duration in patients. Our results reveal that accelerated brain aging occurs at illness onset and suggest it is a stage‐dependent phenomenon in depression.
Keywords: brain age, first‐episode depression, machine learning, structural brain imaging
With the help of prediction model built with two independent datasets, we found stage‐specially accelerated brain aging in patients with depression according to illness duration. Our results reveal that accelerated brain aging occurs at illness onset and suggest it is a stage‐dependent phenomenon in depression.

1. INTRODUCTION
As a common mental disorder, depression that characterized by cognitive and affective deficits (Pan et al., 2018) leads to reduced quality of life and even suicide in patients with depression (S. Han et al., 2019). In addition to affective symptomatology, new evidences suggest that depressed individuals evidently present an increased risk of being attacked by aging‐related somatic diseases (Nicholson, Kuper, & Hemingway, 2006) and mortality (Penninx, 2017) independent of suicide (Nock, Hwang, Sampson, & Kessler, 2010). These evidences include association between depression and decline in cognitive state (John, Patel, & Rusted, 2019), elevated risk of metabolic syndrome (Vancampfort et al., 2014) and cellular aging (Verhoeven et al., 2014) in patients with depression. Recent years, researchers began to use brain images combined with machine learning method to predict brain age (Gaser, Franke, Klöppel, Koutsouleris, & Sauer, 2013; Habes & Janowitz, 2016; Hajek et al., 2019; He et al., 2020) to explore disease such as schizophrenia, mild cognitive impairment (MCI).Exploring whether and how brain aging patterns are altered using machine learning could deepened our understanding of physiological mechanism of these disease.
As one of the brain imaging methods, magnetic resonance imaging (MRI) has the unique ability to noninvasively investigate brain structure and function. Previous structural MRI studies have identified pattern of neuroanatomical change along with normal brain development and aging at the individual level (Bashyam et al., 2020; Jylhävä, Pedersen, & Hägg, 2017). Recently, studies employ machine learning method combined with structural brain MRI images accurately predict individual brain age (Cole, Franke, & Cherbuin, 2019). Then, the prediction model is successfully applied to the study of several neurological diseases and reveals accelerated brain aging in disease such as schizophrenia, MCI, Alzheimer's disease, and autism (Gaser et al., 2013; Habes & Janowitz, 2016; Hajek et al., 2019; He et al., 2020). However, there is a paucity of studies exploring whether and how brain aging patterns is disturbed in patients with depression.
Although accelerated aging trajectories in patients with depression have been supported by mounts of evidences ranging from functional to biological state (Darrow et al., 2016; L. K. M. Han et al., 2018; Lever‐van Milligen, Lamers, Smit, & Penninx, 2017; Lindqvist et al., 2018), the number of studies specifically investing brain aging in depression with structural brain MRI is scarce. What is worse, the findings are not unified (L. K. M. Han, Dinga, Hahn, Ching, & Eyler, 2020). The reason might be the heterogeneity of samples clinical variations such as medicine, recurrence status (first vs. recurrent episode), age of onset, especially for illness duration (Besteher, Gaser, & Nenadić, 2019; L. K. M. Han et al., 2020; Kaufmann & van der Meer, 2019; Koutsouleris et al., 2014). In addition, three main questions remain unsolved in these studies. First, most of patients used in these studies are taking medicine (e.g., antidepressant) at the time of scan that changes brain structure (Cousins & Goodyer, 2015; Hamilton, Siemer, & Gotlib, 2008; Lavretsky, Roybal, Ballmaier, Toga, & Kumar, 2005; Willner, Scheel‐Krüger, & Belzung, 2013), the effect of medicine cannot be completely eliminated. Second, patients enrolled in these studies are mainly Caucasian, whether the findings can be extended to other populations having different genetics, culture and environmental exposures remains unknown (S. Han & Ma, 2014; Nisbett & Miyamoto, 2005; Tang et al., 2018). The last and most important one, no study explores effect of cumulative illness burden on brain aging in depression. Gray matter (GM) volumes are found differently altered at different stages of the illness duration (Bora, Fornito, Pantelis, & Yücel, 2012; Serra‐Blasco et al., 2013) that is thought to be one of the import potential confounders resulting inconsistent findings in depression (Chen et al., 2016). Prospective studies find that major depressive disorder with longer illness duration often experiences increasingly persistent disease with greater proportions of time spent in the midst of a major depressive episode (Judd et al., 1998; Solomon et al., 2000), suggesting neuropathological progression of depression. For example, right hippocampus is found reduced in older patients with depression particularly in patients with a longer course of illness (Bell‐McGinty et al., 2002) and its volume decline is correlated with cumulative illness duration (Sheline, Gado, & Kraemer, 2003). The same progression of GM reduction is observed in rostral anterior cingulate cortex and dorsomedial frontal cortex (Frodl et al., 2008). Unfortunately, there is no study exploring the effect of illness duration on brain aging in depression.
To answer these questions, we constructed a prediction model where GM volumes were treated as features to explore whether brain age is accelerated and its association with clinical characteristics in never‐treated first‐episode patients with depression. Gary matter volumes were quantified using voxel‐based morphometry (VBM). The prediction model was built and validated using HCs in two Chinese Han datasets (Dataset 1, N = 130 for healthy controls [HCs] and N = 195 for patients with depression; Dataset 2, N = 270 for HCs) separately or jointly. Then the trained model was applied to explore brain aging in patients with depression. Brain‐predicted age difference (brain‐PAD) scores defined as the difference between predicted brain age and chronological age, were calculated in patients with depression and then compared with that in age‐, gender‐, educational level‐matched HCs. A positive brain‐PAD reflects accelerated maturation of brain while negative one represents delayed brain development in depression. Based on previous findings, we expected to find higher brain‐PAD scores in patients than that in HCs and explored association between brain‐PAD score and crucial clinical characteristics including illness duration, age of onset and gender.
2. MATERIALS AND METHODS
2.1. Sample
2.1.1. Dataset 1
Patients with depression were recruited from outpatient services of Department of Psychiatry, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China from January 2015 to now. Patients were diagnosed according Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition for depression, this procedure was done by one chief physician and one well‐trained psychiatrist. The following inclusion criteria were employed: (a) the patients with depression must be first episode; (b) never taking any antidepressants (or antipsychotics) and any other antidepressant treatment such as psychological therapy and electric shock treatment; and (c) Han Chinese and right handedness. Patients with depression would be excluded if they met one of the following exclusion criteria: (a) comorbidity of other mental/psychotic disorders and (b) previous episodes of manic symptoms. The clinical states of the patients were evaluated using the 24/17‐items Hamilton Depression (HAMD) scale.
HCs were recruited from the community through poster advertisement. None of them presented a history of serious medical or neuropsychiatric illness or a family history of major psychiatric or neurological illness in their first‐degree relatives. All HCs were Han Chinese and right handedness.
In addition, participants (both HCs and patients) included in the current study must meet the following exclusion criteria: (a) taking drugs such as anesthesia, sleeping, and analgesia in the past 1 month; (b) substance abuse; (c) a history of brain tumor, trauma, surgery, or other organic body disease; (d) suffering from cardiovascular diseases, diabetes, hypertension; (e) contraindications for MRI scanning including fixed dentures, metal braces, artificial heart valves, and other metal foreign bodies in the body; and (f) other structural brain abnormalities revealed by MRI scan.
Written informed consents were obtained from all participants before experiment. The study was approved by the research ethical committee of The First Affiliated Hospital of Zhengzhou University.
2.1.2. Dataset 2
Another dataset come from Southwest University Adult Lifespan Dataset (SALD) study. This dataset was obtained from healthy participants (N = 494, 308 female, 187 male, age range 19–80). The exclusion criteria included MRI‐related exclusion criteria, current psychiatric/neurological disorders, use of psychiatric drugs in the past 3 months prior to scanning and so on. More detailed description about the subject information and data acquisition parameters, please see (Wei et al., 2018), 3D structural MRI sequence was in supplement results. Participants under the age of 50 (N = 270) were included in this study. The data is available for research purposes through the International Data‐sharing Initiative (https://fcon_1000.projects.nitrc.org/indi/retro/sald.html).
2.2. VBM analysis
All scans were processed using the CAT12 toolbox (https://dbm.neuro.uni-jena.de/cat12/). The stand pipeline steps were used including bias‐field correction, segmentation (GM and white matter and cerebrospinal fluid, adjustment for partial volume effects, normalization into Montreal Neurological Institute space, resampled to1.5 mm × 1.5 mm × 1.5 mm and nonlinear modulation (Ashburner, 2009). Finally, the GM maps were smoothed using 6 mm full width at half maximum Gaussian kernel. The total intracranial volume (TIV) of each participant was also calculated for the next comparative analysis.
2.3. Prediction model
The GM volumes of the whole brain were segmented into different regions using brain atlas dividing the whole brain into 268 regions in virtue of a group‐wise spectral clustering algorithm (Finn et al., 2015; Shen, Tokoglu, Papademetris, & Constable, 2013). The mean GM volume of each region was used as features and sent into prediction model. Gaussian process regression (GPR) was used to predict participants' age from the mean GM volumes of all brain regions (Marquand, Rezek, Buitelaar, & Beckmann, 2016; Seeger, 2004). This approach was highly flexible and could accommodate nonlinear relationships delivering state‐of‐the‐art prediction performance in many types of neuroimage data (Hyun et al., 2014). The GPR method was implemented in the Gaussian processes for machine learning (GPML) toolbox (www.gaussianprocess.org/gpml/code/). The model parameters were optimized using a conjugate gradient optimizer (also included in GPML toolbox) as done in before (Marquand et al., 2016; Marquand et al., 2016). We also compared the performance of different machine learning methods commonly used in previous studies (He et al., 2020; Koutsouleris et al., 2014; Sone et al., 2019). Specially, we compared the prediction performance of different machine learners (GPR vs. support vector regression [SVR]) and feature selection strategies (mean GM volumes based on atlas vs. principal component analysis [PCA] (Franke, Ziegler, Klöppel, & Gaser, 2010)). When PCA was used, the first N eigenvariates explaining the 95% of variance were extracted as features in the training process.
2.4. Model validation
Ten folds cross‐validation (CV) were used to evaluate the performance of the prediction model (Sone et al., 2019; ZIEGEL, 2010). This procedure divided the samples into 10 subsets of (approximately) equal size. We trained the model 10 times where one of the subsets was treated as test sample set and the others train sample each time. The trained model learned from the train sample set was then applied to the test sample to obtain bran age. We calculated (a) mean absolute error (MAE) between estimated brain age (output of the prediction model) and chronological age and (b) the correlation between the chronological age and estimated brain age through 10‐fold CV.
In this procedure, two datasets were used separately or jointly to evaluate the performance and universality of our prediction model. That is to say, 10‐fold CV was done in dataset 1 (HCs, N = 130), dataset 2 (N = 270) and a collection of both datasets (HCs in Dataset 1 and all participants in dataset 2, N = 400) to evaluate the performance of the prediction model.
To eliminate the effect of chronological age on the statistical results of altered brain‐PAD scores in patients as much as possible, we only included participants under age of 36 (N = 182) in Dataset 2 in the final prediction model built to compare brain‐PAD scores between HCs and patients because that the brain‐PAD scores were found to be highly related with chronological age (Hajek et al., 2019; L. K. M. Han et al., 2018; L. K. M. Han et al., 2020). Based on the prediction model, we calculated each participant's brain‐PAD (score: predicted age–chronological age).
2.5. Statistics
The brain‐PAD scores were compared between patients with depression and HCs in Dataset 1 using two‐tailed two‐sample t test. Gender, age, and age2 were used as covariates in statistical models (L. K. M. Han et al., 2020). Moreover, we also divided patients with depression into three stages according to illness duration (Stage 1:0–12 months; Stage 2:12–24 months; Stage 3: > = 24 months) to explore whether aberrance of brain‐PAD scores was stage dependent, because mental disorders including depression were found to be related with progressive brain structural alterations (Cao, Passos, Mwangi, Amaral‐Silva, & Tannous, 2017; Koutsouleris et al., 2014; Treadway et al., 2015; Yüksel et al., 2018; Zhang et al., 2017). At the same time, to explore whether the altered brain‐PAD scores occurred at illness onset, we also compared brain‐PAD scores in patients with much shorted illness duration (<3/6 months) with that in matched HCs. All above‐mentioned statistical procedures were done between patients with depression and age‐, gender‐, and educational level‐matched HCs in Dataset 1 and statistical results were adjusted for gender, age, and age2.
Meanwhile, a newly proposed data‐driven algorithm (HYDRA) (Chand et al., 2020; Varol, Sotiras, & Davatzikos, 2017) was used to divide patients with depression into subgroups according to structural GM volumes that used in the prediction model (details in supplement results). This procedure was done to validate stage‐specific aberrance of brain‐PAD scores in patients from the opposite direction. That is to say, if brain‐PAD scores were stage‐specifically altered, patients would be divided into subgroups showing significant difference of illness duration. HYDRA could perform classification and subtyping simultaneously where classification was performed through the separation of HCs from patients with depression and subtyping was carried out by clustering patients with hyperplanes. The number of subgroups was set 1 to 5. Finally, we explored difference of clinical variables including gender, age, age of first onset, and illness duration among subgroups.
Within patients with depression, we also consider the effect of factors such as gender (male vs. female), age of onset of depression (categorized as: early/adolescent onset, < = 25 years (Penttilä et al., 2009; Truong et al., 2013) (or 21 years (Schmaal et al., 2016; Schmaal et al., 2017)) years; middle adulthood/adult onset, >25 years (or 21) years) on the brain‐PAD scores. Especially, to explore the difference of brain‐PAD scores between male and female patients, we built prediction model using male and female HCs jointly or separately. In this procedure, patients were divided into two subgroups according to factors (male vs. female or adolescent onset vs. adult onset). Then, brain‐PAD scores were compared between subgroups using two sample t test adjusted for gender (not for gender difference), age and age2. We also compared the TIV of patients with that of HCs at each stage and across stages.
To explore the relationship between brain‐PAD scores and clinical variables, we calculated Spearman correlations between brain‐PAD scores and the total HAMD score and illness duration.
3. RESULTS
3.1. Clinical demographics
The clinical demographics are presented in Table 1. The distribution of participants' age is drawn in Figure 1. The clinical demographics of patients at different stages and matched HCs were in Table S1.
TABLE 1.
Demographic and clinical characteristics of participants
| Dataset 1 | Dataset 2 | ||
|---|---|---|---|
| HC (N = 130) | Depression (N = 195) | Subjects (N = 270) | |
| Male, No. (%) | 59 (45.38) | 95 (48.7) | 98 (36.30) |
| Age, mean (SD) [range], years | 21.25 (5.33) [12–36] | 18.14 (4.47) [11–37] | 31.50 (9.99) [19–50] |
| Educational level, mean (SD), years | 13.56 (4.50) | 10.11 (2.13) | — |
| Duration of illness, mean (SD), months | — | 15.74 (16.96) | — |
| HAMD score, mean (SD), [range] | — |
22.38 (5.72) [12–48] a 39.29 (11.68) [20–61] b |
— |
| Handedness, right/left | 130/0 | 195/0 | — |
| Age of first onset, years | — | 16.81 (4.40) | — |
Abbreviations: HAMD, Hamilton rating scale for depression, HC, healthy controls.
17‐items HAMD for 167 patients.
24‐items HAMD for 28 patients.
FIGURE 1.
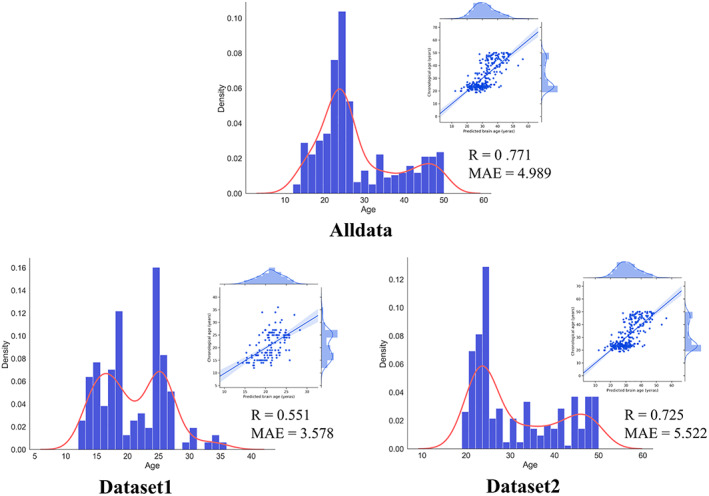
The distribution of age in datasets and the performance of the proposed prediction model. R: Pearson's correlation; MAE, mean absolute error
3.2. Prediction model performance in HCs
Within healthy participants, our proposed prediction model presented steady performance across datasets (Figure 1). We also found performance of the prediction model was better when the sample size was larger. This result was in line with the conclusion of previous study (Franke et al., 2010). In addition, we compared our proposed prediction model with other models commonly used in previous studies. As a result, the prediction model used in the current study presented overall better performance than other methods (Table S2).
3.3. Brain‐PAD scores in patients with depression
Overall, patients with depression presented higher brain‐PAD scores than that in matched HCs (+0.586 years, t = 2.065, p = 0.039, Cohen'd = 0.277) adjusted for gender, age, and age2.
Then, we divided patients into three stages according to illness duration and compared their brain‐PAD scores with that in matched HCs. We found that only at the first two stage (Stage 1, +0.812 years, t = 2.639, p = .009, d = 0.413; Stage 2, +0.742 years, t = 2.056, p = .042, d = 0.365) presented significant higher brain‐PAD scores than HCs, while patients with depression at Dtage 3 (illness duration > = 24 months) did not (t = 1.507, p = .134, d = 0.270). We further explored whether brain‐PAD scores were higher in depression at illness onset. Brain‐PAD scores of patients whose illness duration was <3/6 months were compared with that in matched HCs. As a result, we found that patients with depression presented higher brain‐PAD scores than that in HCs at the beginning of illness (<3 months, +1.061 years, t = 2.520, p = .013, d = 0.546; <6 months, +0.942 years, t = 2.829, p = .005, d = 0.505) (Figure 2).
FIGURE 2.
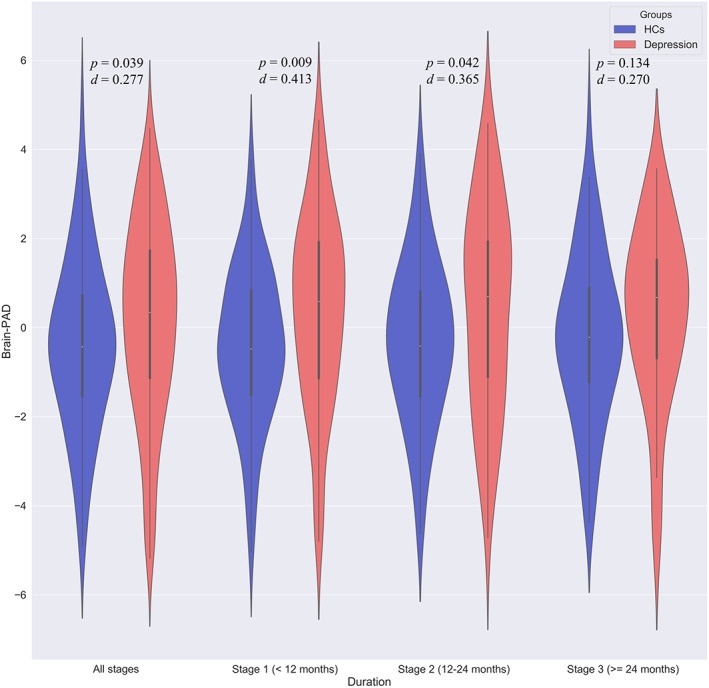
Aberrance of brain‐predicted age difference (brain‐PAD) scores in patients at different stages adjusted for gender, age, and age2
As a complement to these results, we employed a data‐driven method dividing patients into subgroups and then compared clinical variables among subgroups. The largest adjusted rand index (ARI) was found at K = 2 (ARI = 0.885) (Figure S1 in supplement results), suggesting patients with depression could be divided into two distinct subgroups. Then, we compared clinical variables including gender, age, age of first onset and illness duration between two subgroups. As a result, only illness duration presented significant difference between two subgroups (t = −2.101, p = .0367, d = −0.301) (Figure S2 in supplement results) (Figure 3).
FIGURE 3.
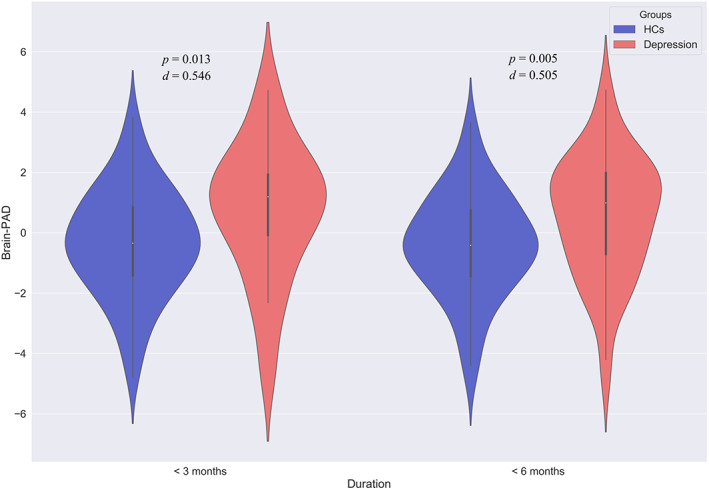
Aberrance of brain‐predicted age difference (brain‐PAD) scores in patients at illness onset (<3/6 months) adjusted for gender, age, and age2
There was no not significant difference of brain‐PAD scores between female and male patients with depression, all p values >.05 in two different prediction models constructed using female and male participants separately or jointly. Performance of the prediction model using female and male participants separately was drawn in Figure S3 (Supplement results). We also did not find significant effect of age of onset on the brain‐PAD scores in patients with depression.
Meanwhile, brain‐PAD scores were significant correlated with illness duration in patients with depression (Spearman R = −1.145, p = .043). There was no significant correlation between brain‐PAD scores with the total score of HAMD. We did not observe significant difference of TIV between patients and HCs at each stage and across stages (Figure 4).
FIGURE 4.
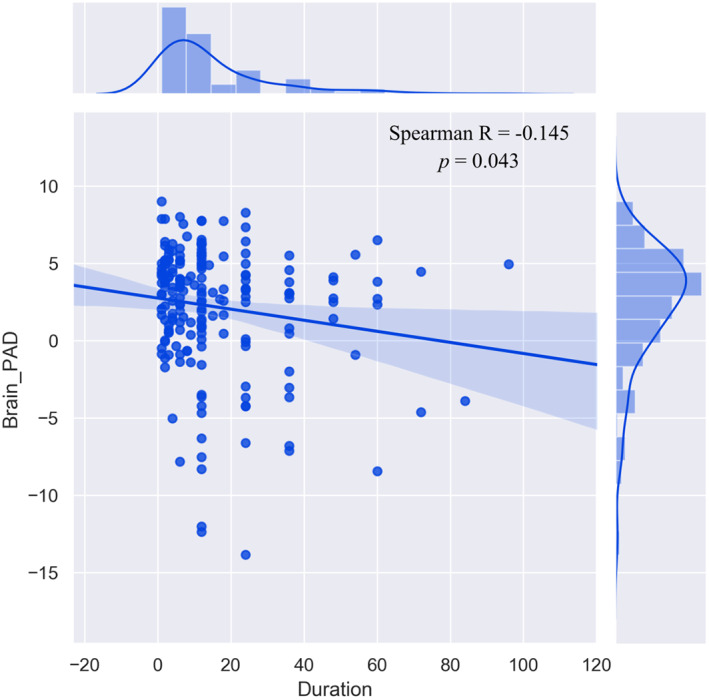
The correlation between brain‐predicted age difference (brain‐PAD) scores and illness duration in patients with depression
4. DISCUSSION
Patients with depression presented higher brain‐PAD scores than HCs suggesting patients with depression having an “older” brain than expected. This phenomenon occurred since the beginning of the illness (<3/6 months) and lasted for up to the first 2 years of illness. Meanwhile, results of HYDRA revealed two distinct subgroups of patients presenting characterized with different length of illness duration. Brain‐PAD scores were negatively correlated with illness duration in patients with depression. These converging results confirmed accelerated brain aging occurred at illness onset and hinted it was a stage‐dependent phenomenon in depression.
Our proposed prediction model presented stable performance across highly heterogeneous datasets having different scanning parameters and age distribution. Consistent with the conclusion of previous study focusing on influence of various parameters on performance of prediction model (Franke et al., 2010), we also found that the performance of prediction model got better as the sample size increased in the results. The MAE (4.990 year in age range of 11–50 years, 0.128 after scaled to covered age range) of our proposed model built using all HCs across two datasets (N = 400) was close to that reported in previous studies (L. K. M. Han et al., 2020; Schnack et al., 2016). The comparatively large MAE in our results might be ascribed more to relatively small sample size. In addition, we compared different machine learners (GPR vs. SVR) and feature selection strategies (mean GM volumes based on atlas vs. PCA), the results illustrated that our proposed model had obvious better performance (Table S1 in supplement results) suggesting GPR was highly flexible delivering state‐of‐the‐art prediction performance in many types of neuroimage data (Hyun et al., 2014). A more elaborate methodological comparison was omitted because it was beyond the scope of the current study.
As we hypothesized, brain‐PAD scores were higher (+0.586 years for all, ranging from +0.742 to +1.061 years for different stages) in never‐treated first‐episode patients with depression than that in HCs. These results replicated earlier results (L. K. M. Han et al., 2020; Koutsouleris et al., 2014) in Chinese Han patients with depression suggesting the higher brain‐PAD score in depression was not about race having different genetics, culture and environmental exposures. Higher brain‐PAD scores suggesting accelerated aging trajectories were related to greater cognitive impairment in depression (Hatton et al., 2018). One proposed explanation was the common biological mechanism underlying depression and brain aging (Franceschi et al., 2000; L. K. M. Han et al., 2020), supported by findings that brain‐PAD was temporarily reduced by 1.1 years introduced by the probable acute anti‐inflammatory effects of ibuprofen in HCs (Le et al., 2018). Moreover, brain‐PAD scores were higher even at illness onset (with illness duration <3 months) and not associated with clinical variations including gender and onset of illness in depression, hinting it was related to pathomechanism of depression not the consequence of illness progression. Brain‐PAD difference in our results was closer to that (+1.1 years) reported in Han et al. (L. K. M. Han et al., 2020), the effect size of difference was much higher than that reported before (L. K. M. Han et al., 2020), the reason might be that we only enrolled never‐treated first‐episode patients in the current study excluding effect of clinical variations such as medication status and acquisition protocols. Although the relatively smaller brain‐PAD scores might partly come from factors including age range and distribution, scanning parameters and sample size, the more likely reason might be that higher brain age was a stage‐dependent phenomenon in depression.
The difference of brain‐PAD scores were initially prominent and then become less pronounced after 2 years of illness in patients, hinting higher brain age was a stage‐dependent phenomenon in depression. Mounts of evidences suggested depression was a neuroprogressive illness (Moylan, Maes, Wray, & Berk, 2013). Longer and more frequent depressive episodes were accompanied by increase vulnerability to further relapses and functional decline (Kendler, Thornton, & Gardner, 2001). In a recent review (Moylan et al., 2013), the authors collected evidences covering from clinical, biochemical and neuroimaging studies and indicated neuroprogressive process occurring in depression. For example, GM volumes of the hippocampus and medial frontal cortex were associated with greater number of prior depressive episodes (Belleau, Treadway, & Pizzagalli, 2019), resulted from chronic stress and toxic effects of recurrent depressive episodes (Treadway et al., 2015) and the same reason leading to progressive anterior cingulate cortex and dorsomedial frontal changes in depression (Bora et al., 2012). In the latest research of Elaine et al., the authors found strong correlation between increasing duration of untreated illness and greater microglial activation, providing a new evidence of neuroprogression (Setiawan et al., 2018). Consistent with these studies, we found the differences of brain‐PAD scores between patients with depression and HCs were stage‐dependent. Specially, the difference of brain‐PAD scores between patients and HCs were initially high and then become less pronounced after 2 years of illness in patients with depression. This phenomenon could mirror the transition from a clinically unstable period, with large variability in functioning, to a relatively stable period, when patients have reached a plateau in functioning (Davidson & McGlashan, 1997; van Haren et al., 2003). Notably, unsignificant brain‐PAD scores in patients with longer illness duration (> = 2 years) did not necessarily declare the remission of severity of illness, we did observe significant difference of the total score of HAMD (p = .185, t = 1.331) between patients with longer duration (> = 2 years) and ones with shorter illness duration (<2 years). One reason of Bianca et al. failing to find accelerated aging in patients with depression might be illness duration of patients recruited was too long (Besteher et al., 2019). Although the illness duration of patients was not reported, the most of patients used in their study were experiencing multiple episodes tending to have a longer course of illness. Unfortunately, there were rare studies having complete information on the cumulative illness duration across episodes that was different from the index episode being a recurrent or first‐onset episode (Ten Have et al., 2017), given the relapsing and remitting nature of depression (Schmaal et al., 2016). More longitudinal studies with first‐episode patients (or accurately recording cumulative disease burden) were needed to validate this assumption. In addition, we also found that patients were divided into two subgroups only having significantly different length of illness duration with data‐driven algorithm (HYDRA). This result was consistent with another study also finding illness duration was an important factor varied between two subgroups revealed by cluster analysis (Corponi & Anmella, 2020). Previous studies also found patients with longer illness duration were accompanied by greater severity (Spijker et al., 2002; Ten Have et al., 2017), higher comorbidities' burden, suicide behavior (Spijker et al., 2002) and lower probability of recovery (Corponi & Anmella, 2020; Keller et al., 1992). Taken together, these findings convergently hinted accelerated brain aging was also related to illness duration and a stage‐dependent phenomenon in depression.
There were several limitations must be considered. First, patients all come from a single dataset, another independent dataset was needed to validate our results in the future. Second, patient enrolled in the current study were under depressive state, whether the accelerated brain aging was trait related or state related should be tested with patients under the remitted state in future study (Rive et al., 2015; Van Eijndhoven et al., 2013). Third, the age of patients was less than 36 years, whether the conclusion held true for late‐onset depression needed to be explored (Penttilä et al., 2009; Truong et al., 2013).
5. CONCLUSION
In the current study, we enrolled Chinese Han patients to confirm accelerated brain aging and explore effects of clinical variations on the brain age in depression. Overall, patients presented higher brain‐PAD scores suggesting patients with depression having an “older” brain than expected. This difference occurred in patients at illness onset (illness duration <3 months) and following 2 years then disappeared as the illness further advances (>2 years). In addition, we found that patients were divided into two subgroups characterized with length of illness duration, by data‐driven method employed to explore factors affecting brain‐PAD scores and validate our results. Combing with the correlation between brain‐PAD scores and illness duration in patients, these converging results confirmed higher brain age and hinted accelerated brain aging was a stage‐dependent phenomenon in depression.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
Supporting information
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
The authors thank all subjects who participate in this study. This research study was supported by the Natural Science Foundation of China (81601467, 81871327) and Medical Science and Technology Research Project of Henan Province (201701011).
Han S, Chen Y, Zheng R, et al. The stage‐specifically accelerated brain aging in never‐treated first‐episode patients with depression. Hum Brain Mapp. 2021;42:3656–3666. 10.1002/hbm.25460
Shaoqiang Han and Yuan Chen contributed equally to this study.
Funding information Medical Science and Technology Research Project of Henan Province; Natural Science Foundation of China, Grant/Award Numbers: 201701011, 81871327, 81601467
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Ashburner, J. (2009). Computational anatomy with the SPM software. Magnetic Resonance Imaging, 27(8), 1163–1174. 10.1016/j.mri.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Bashyam, V. M. , Erus, G. , Doshi, J. , Habes, M. , Nasralah, I. , Truelove‐Hill, M. , … Davatzikos, C. (2020). MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain, 143(7), 2312–2324. 10.1093/brain/awaa160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau, E. L. , Treadway, M. T. , & Pizzagalli, D. A. (2019). The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biological Psychiatry, 85(6), 443–453. 10.1016/j.biopsych.2018.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell‐McGinty, S. , Butters, M. A. , Meltzer, C. C. , Greer, P. J. , Reynolds, C. F., III , & Becker, J. T. (2002). Brain morphometric abnormalities in geriatric depression: Long‐term neurobiological effects of illness duration. The American Journal of Psychiatry, 159(8), 1424–1427. 10.1176/appi.ajp.159.8.1424 [DOI] [PubMed] [Google Scholar]
- Besteher, B. , Gaser, C. , & Nenadić, I. (2019). Machine‐learning based brain age estimation in major depression showing no evidence of accelerated aging. Psychiatry Research: Neuroimaging, 290, 1–4. 10.1016/j.pscychresns.2019.06.001 [DOI] [PubMed] [Google Scholar]
- Bora, E. , Fornito, A. , Pantelis, C. , & Yücel, M. (2012). Gray matter abnormalities in major depressive disorder: A meta‐analysis of voxel based morphometry studies. Journal of Affective Disorders, 138(1–2), 9–18. 10.1016/j.jad.2011.03.049 [DOI] [PubMed] [Google Scholar]
- Cao, B. , Passos, I. C. , Mwangi, B. , Amaral‐Silva, H. , & Tannous, J. (2017). Hippocampal subfield volumes in mood disorders. Molecular Psychiatry, 22(9), 1352–1358. 10.1038/mp.2016.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand, G. B. , Dwyer, D. B. , Erus, G. , Sotiras, A. , Varol, E. , Srinivasan, D. , … Davatzikos, C. (2020). Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain, 143(3), 1027–1038. 10.1093/brain/awaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Peng, W. , Sun, H. , Kuang, W. , Li, W. , Jia, Z. , & Gong, Q. (2016). High‐field magnetic resonance imaging of structural alterations in first‐episode, drug‐naive patients with major depressive disorder. Translational Psychiatry, 6(11), e942. 10.1038/tp.2016.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J. H. , Franke, K. , & Cherbuin, N. (2019). Quantification of the biological age of the brain using neuroimaging. In Biomarkers of human aging. Switzerland: Springer; (pp. 293–328). [Google Scholar]
- Corponi, F. , & Anmella, G. (2020). Deconstructing major depressive episodes across unipolar and bipolar depression by severity and duration: A cross‐diagnostic cluster analysis on a large, international, observational study. Translational Psychiatry, 10(1), 241. 10.1038/s41398-020-00922-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins, L. , & Goodyer, I. M. (2015). Antidepressants and the adolescent brain. Journal of Psychopharmacology, 29(5), 545–555. 10.1177/0269881115573542 [DOI] [PubMed] [Google Scholar]
- Darrow, S. M. , Verhoeven, J. E. , Révész, D. , Lindqvist, D. , Penninx, B. W. , Delucchi, K. L. , … Mathews, C. A. (2016). The association between psychiatric disorders and telomere length: A meta‐analysis involving 14,827 persons. Psychosomatic Medicine, 78(7), 776–787. 10.1097/psy.0000000000000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, L. , & McGlashan, T. H. (1997). The varied outcomes of schizophrenia. Canadian Journal of Psychiatry, 42(1), 34–43. 10.1177/070674379704200105 [DOI] [PubMed] [Google Scholar]
- Finn, E. S. , Shen, X. , Scheinost, D. , Rosenberg, M. D. , Huang, J. , Chun, M. M. , … Constable, R. T. (2015). Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nature Neuroscience, 18(11), 1664–1671. 10.1038/nn.4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi, C. , Bonafè, M. , Valensin, S. , Olivieri, F. , De Luca, M. , Ottaviani, E. , & De Benedictis, G. (2000). Inflamm‐aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences, 908, 244–254. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- Franke, K. , Ziegler, G. , Klöppel, S. , & Gaser, C. (2010). Estimating the age of healthy subjects from T1‐weighted MRI scans using kernel methods: Exploring the influence of various parameters. NeuroImage, 50(3), 883–892. 10.1016/j.neuroimage.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Frodl, T. S. , Koutsouleris, N. , Bottlender, R. , Born, C. , Jäger, M. , Scupin, I. , … Meisenzahl, E. M. (2008). Depression‐related variation in brain morphology over 3 years: Effects of stress? Archives of General Psychiatry, 65(10), 1156–1165. 10.1001/archpsyc.65.10.1156 [DOI] [PubMed] [Google Scholar]
- Gaser, C. , Franke, K. , Klöppel, S. , Koutsouleris, N. , & Sauer, H. (2013). BrainAGE in mild cognitive impaired patients: Predicting the conversion to Alzheimer's disease. PLoS One, 8(6), e67346. 10.1371/journal.pone.0067346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habes, M. , & Janowitz, D. (2016). Advanced brain aging: Relationship with epidemiologic and genetic risk factors, and overlap with Alzheimer disease atrophy patterns. Translational Psychiatry, 6(4), e775. 10.1038/tp.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, T. , Franke, K. , Kolenic, M. , Capkova, J. , Matejka, M. , Propper, L. , … Alda, M. (2019). Brain age in early stages of bipolar disorders or schizophrenia. Schizophrenia Bulletin, 45(1), 190–198. 10.1093/schbul/sbx172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, J. P. , Siemer, M. , & Gotlib, I. H. (2008). Amygdala volume in major depressive disorder: A meta‐analysis of magnetic resonance imaging studies. Molecular Psychiatry, 13(11), 993–1000. 10.1038/mp.2008.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, L. K. M. , Aghajani, M. , Clark, S. L. , Chan, R. F. , Hattab, M. W. , Shabalin, A. A. , … Penninx, B. (2018). Epigenetic aging in major depressive disorder. The American Journal of Psychiatry, 175(8), 774–782. 10.1176/appi.ajp.2018.17060595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, L. K. M. , Dinga, R. , Hahn, T. , Ching, C. R. K. , & Eyler, L. T. (2020). Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Molecular Psychiatry. 10.1038/s41380-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S. , & Ma, Y. (2014). Cultural differences in human brain activity: A quantitative meta‐analysis. NeuroImage, 99, 293–300. 10.1016/j.neuroimage.2014.05.062 [DOI] [PubMed] [Google Scholar]
- Han, S. , Wang, X. , He, Z. , Sheng, W. , Zou, Q. , Li, L. , … Chen, H. (2019). Decreased static and increased dynamic global signal topography in major depressive disorder. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 94, 109665. 10.1016/j.pnpbp.2019.109665 [DOI] [PubMed] [Google Scholar]
- Hatton, S. N. , Franz, C. E. , Elman, J. A. , Panizzon, M. S. , Hagler, D. J., Jr. , Fennema‐Notestine, C. , … Kremen, W. S. (2018). Negative fateful life events in midlife and advanced predicted brain aging. Neurobiology of Aging, 67, 1–9. 10.1016/j.neurobiolaging.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, C. , Chen, H. , Uddin, L. Q. , Erramuzpe, A. , Bonifazi, P. , Guo, X. , … Duan, X. (2020). Structure‐function connectomics reveals aberrant developmental trajectory occurring at preadolescence in the autistic brain. Cerebral Cortex, 30(9), 5028–5037. 10.1093/cercor/bhaa098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun, J. W. , Li, Y. , Gilmore, J. H. , Lu, Z. , Styner, M. , & Zhu, H. (2014). SGPP: Spatial Gaussian predictive process models for neuroimaging data. NeuroImage, 89, 70–80. 10.1016/j.neuroimage.2013.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, A. , Patel, U. , & Rusted, J. (2019). Affective problems and decline in cognitive state in older adults: A systematic review and meta‐analysis. Psychological Medicine, 49(3), 353–365. 10.1017/s0033291718001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd, L. L. , Akiskal, H. S. , Maser, J. D. , Zeller, P. J. , Endicott, J. , Coryell, W. , … Keller, M. B. (1998). A prospective 12‐year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Archives of General Psychiatry, 55(8), 694–700. 10.1001/archpsyc.55.8.694 [DOI] [PubMed] [Google Scholar]
- Jylhävä, J. , Pedersen, N. L. , & Hägg, S. (2017). Biological age predictors. eBioMedicine, 21, 29–36. 10.1016/j.ebiom.2017.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann, T. , & van der Meer, D. (2019). Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nature Neuroscience, 22(10), 1617–1623. 10.1038/s41593-019-0471-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, M. B. , Lavori, P. W. , Mueller, T. I. , Endicott, J. , Coryell, W. , Hirschfeld, R. M. , & Shea, T. (1992). Time to recovery, chronicity, and levels of psychopathology in major depression. A 5‐year prospective follow‐up of 431 subjects. Archives of General Psychiatry, 49(10), 809–816. 10.1001/archpsyc.1992.01820100053010 [DOI] [PubMed] [Google Scholar]
- Kendler, K. S. , Thornton, L. M. , & Gardner, C. O. (2001). Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. The American Journal of Psychiatry, 158(4), 582–586. 10.1176/appi.ajp.158.4.582 [DOI] [PubMed] [Google Scholar]
- Koutsouleris, N. , Davatzikos, C. , Borgwardt, S. , Gaser, C. , Bottlender, R. , Frodl, T. , … Meisenzahl, E. (2014). Accelerated brain aging in schizophrenia and beyond: A neuroanatomical marker of psychiatric disorders. Schizophrenia Bulletin, 40(5), 1140–1153. 10.1093/schbul/sbt142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky, H. , Roybal, D. J. , Ballmaier, M. , Toga, A. W. , & Kumar, A. (2005). Antidepressant exposure may protect against decrement in frontal gray matter volumes in geriatric depression. The Journal of Clinical Psychiatry, 66(8), 964–967. 10.4088/jcp.v66n0801 [DOI] [PubMed] [Google Scholar]
- Le, T. T. , Kuplicki, R. , Yeh, H. W. , Aupperle, R. L. , Khalsa, S. S. , Simmons, W. K. , & Paulus, M. P. (2018). Effect of Ibuprofen on BrainAGE: a randomized, placebo‐controlled, dose‐response exploratory study. Biological psychiatry . Cognitive Neuroscience and Neuroimaging, 3(10), 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever‐van Milligen, B. A. , Lamers, F. , Smit, J. H. , & Penninx, B. W. (2017). Six‐year trajectory of objective physical function in persons with depressive and anxiety disorders. Depression and Anxiety, 34(2), 188–197. 10.1002/da.22557 [DOI] [PubMed] [Google Scholar]
- Lindqvist, D. , Wolkowitz, O. M. , Picard, M. , Ohlsson, L. , Bersani, F. S. , Fernström, J. , … Mellon, S. H. (2018). Circulating cell‐free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology, 43(7), 1557–1564. 10.1038/s41386-017-0001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquand, A. F. , Rezek, I. , Buitelaar, J. , & Beckmann, C. F. (2016). Understanding heterogeneity in clinical cohorts using normative models: Beyond case‐control studies. Biological Psychiatry, 80(7), 552–561. 10.1016/j.biopsych.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan, S. , Maes, M. , Wray, N. R. , & Berk, M. (2013). The neuroprogressive nature of major depressive disorder: Pathways to disease evolution and resistance, and therapeutic implications. Molecular Psychiatry, 18(5), 595–606. 10.1038/mp.2012.33 [DOI] [PubMed] [Google Scholar]
- Nicholson, A. , Kuper, H. , & Hemingway, H. (2006). Depression as an aetiologic and prognostic factor in coronary heart disease: A meta‐analysis of 6362 events among 146 538 participants in 54 observational studies. European Heart Journal, 27(23), 2763–2774. 10.1093/eurheartj/ehl338 [DOI] [PubMed] [Google Scholar]
- Nisbett, R. E. , & Miyamoto, Y. (2005). The influence of culture: Holistic versus analytic perception. Trends in Cognitive Sciences, 9(10), 467–473. 10.1016/j.tics.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Nock, M. K. , Hwang, I. , Sampson, N. A. , & Kessler, R. C. (2010). Mental disorders, comorbidity and suicidal behavior: Results from the National Comorbidity Survey Replication. Molecular Psychiatry, 15(8), 868–876. 10.1038/mp.2009.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, J. X. , Xia, J. J. , Deng, F. L. , Liang, W. W. , Wu, J. , Yin, B. M. , … Xie, P. (2018). Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: A targeted metabolomics study. Translational Psychiatry, 8(1), 130. 10.1038/s41398-018-0183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx, B. W. (2017). Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neuroscience & Biobehavioral Reviews, 74(Pt B), 277–286. 10.1016/j.neubiorev.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Penttilä, J. , Cachia, A. , Martinot, J. L. , Ringuenet, D. , Wessa, M. , Houenou, J. , … Paillère‐Martinot, M. L. (2009). Cortical folding difference between patients with early‐onset and patients with intermediate‐onset bipolar disorder. Bipolar Disorders, 11(4), 361–370. 10.1111/j.1399-5618.2009.00683.x [DOI] [PubMed] [Google Scholar]
- Rive, M. M. , Mocking, R. J. T. , Koeter, M. W. J. , Wingen, G. V. , Wit, S. J. D. , Heuvel, O. A. V. D. , … Schene, A. H. (2015). State‐dependent differences in emotion regulation between unmedicated bipolar disorder and major depressive disorder. JAMA Psychiatry, 72(7), 687–696. [DOI] [PubMed] [Google Scholar]
- Schmaal, L. , Hibar, D. P. , Sämann, P. G. , Hall, G. B. , Baune, B. T. , Jahanshad, N. , … Tiemeier, H. (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular Psychiatry, 22(6), 900–909. 10.1038/mp.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Veltman, D. J. , van Erp, T. G. , Sämann, P. G. , Frodl, T. , Jahanshad, N. , … Tiemeier, H. (2016). Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder Working Group. Molecular Psychiatry, 21(6), 806–812. 10.1038/mp.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack, H. G. , van Haren, N. E. , Nieuwenhuis, M. , Hulshoff Pol, H. E. , Cahn, W. , & Kahn, R. S. (2016). Accelerated brain aging in schizophrenia: A longitudinal pattern recognition study. The American Journal of Psychiatry, 173(6), 607–616. 10.1176/appi.ajp.2015.15070922 [DOI] [PubMed] [Google Scholar]
- Seeger, M. (2004). Gaussian processes for machine learning. International Journal of Neural Systems, 14(2), 69–106. 10.1142/s0129065704001899 [DOI] [PubMed] [Google Scholar]
- Serra‐Blasco, M. , Portella, M. J. , Gómez‐Ansón, B. , de Diego‐Adeliño, J. , Vives‐Gilabert, Y. , Puigdemont, D. , … Pérez, V. (2013). Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. The British Journal of Psychiatry, 202, 434–440. 10.1192/bjp.bp.112.116228 [DOI] [PubMed] [Google Scholar]
- Setiawan, E. , Attwells, S. , Wilson, A. A. , Mizrahi, R. , Rusjan, P. M. , Miler, L. , … Meyer, J. H. (2018). Association of translocator protein total distribution volume with duration of untreated major depressive disorder: A cross‐sectional study. Lancet Psychiatry, 5(4), 339–347. 10.1016/s2215-0366(18)30048-8 [DOI] [PubMed] [Google Scholar]
- Sheline, Y. I. , Gado, M. H. , & Kraemer, H. C. (2003). Untreated depression and hippocampal volume loss. The American Journal of Psychiatry, 160(8), 1516–1518. 10.1176/appi.ajp.160.8.1516 [DOI] [PubMed] [Google Scholar]
- Shen, X. , Tokoglu, F. , Papademetris, X. , & Constable, R. T. (2013). Groupwise whole‐brain parcellationss from resting‐state fMRI data for network node identification. NeuroImage, 82, 403–41s5. 10.1016/j.neuroimage.2013.05.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, D. A. , Keller, M. B. , Leon, A. C. , Mueller, T. I. , Lavori, P. W. , Shea, M. T. , … Endicott, J. (2000). Multiple recurrences of major depressive disorder. The American Journal of Psychiatry, 157(2), 229–233. 10.1176/appi.ajp.157.2.229 [DOI] [PubMed] [Google Scholar]
- Sone, D. , Beheshti, I. , Maikusa, N. , Ota, M. , Kimura, Y. , Sato, N. , … Matsuda, H. (2019). Neuroimaging‐based brain‐age prediction in diverse forms of epilepsy: A signature of psychosis and beyond. Molecular Psychiatry, 26, 825–834. 10.1038/s41380-019-0446-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker, J. , de Graaf, R. , Bijl, R. V. , Beekman, A. T. , Ormel, J. , & Nolen, W. A. (2002). Duration of major depressive episodes in the general population: Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). The British Journal of Psychiatry, 181, 208–213. 10.1192/bjp.181.3.208 [DOI] [PubMed] [Google Scholar]
- Tang, Y. , Zhao, L. , Lou, Y. , Shi, Y. , Fang, R. , Lin, X. , … Toga, A. (2018). Brain structure differences between Chinese and Caucasian cohorts: A comprehensive morphometry study. Human Brain Mapping, 39(5), 2147–2155. 10.1002/hbm.23994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Have, M. , Penninx, B. , Tuithof, M. , van Dorsselaer, S. , Kleinjan, M. , Spijker, J. , & de Graaf, R. (2017). Duration of major and minor depressive episodes and associated risk indicators in a psychiatric epidemiological cohort study of the general population. Acta Psychiatrica Scandinavica, 136(3), 300–312. 10.1111/acps.12753 [DOI] [PubMed] [Google Scholar]
- Treadway, M. T. , Waskom, M. L. , Dillon, D. G. , Holmes, A. J. , Park, M. T. M. , Chakravarty, M. M. , … Pizzagalli, D. A. (2015). Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biological Psychiatry, 77(3), 285–294. 10.1016/j.biopsych.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong, W. , Minuzzi, L. , Soares, C. N. , Frey, B. N. , Evans, A. C. , MacQueen, G. M. , & Hall, G. B. (2013). Changes in cortical thickness across the lifespan in major depressive disorder. Psychiatry Research, 214(3), 204–211. 10.1016/j.pscychresns.2013.09.003 [DOI] [PubMed] [Google Scholar]
- van Eijndhoven, P. , van Wingen, G. , Katzenbauer, M. , Groen, W. , Tepest, R. , Fernández, G. , … Tendolkar, I. (2013). Paralimbic cortical thickness in first‐episode depression: Evidence for trait‐related differences in mood regulation. American Journal of Psychiatry, 170(12), 1477–1486. [DOI] [PubMed] [Google Scholar]
- van Haren, N. E. , Cahn, W. , Hulshoff Pol, H. E. , Schnack, H. G. , Caspers, E. , Lemstra, A. , … Kahn, R. S. (2003). Brain volumes as predictor of outcome in recent‐onset schizophrenia: A multi‐center MRI study. Schizophrenia Research, 64(1), 41–52. 10.1016/s0920-9964(03)00018-5 [DOI] [PubMed] [Google Scholar]
- Vancampfort, D. , Correll, C. U. , Wampers, M. , Sienaert, P. , Mitchell, A. J. , de Herdt, A. , … de Hert, M. (2014). Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: A meta‐analysis of prevalences and moderating variables. Psychological Medicine, 44(10), 2017–2028. 10.1017/s0033291713002778 [DOI] [PubMed] [Google Scholar]
- Varol, E. , Sotiras, A. , & Davatzikos, C. (2017). HYDRA: Revealing heterogeneity of imaging and genetic patterns through a multiple max‐margin discriminative analysis framework. NeuroImage, 145(Pt B), 346–364. 10.1016/j.neuroimage.2016.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven, J. E. , Révész, D. , Epel, E. S. , Lin, J. , Wolkowitz, O. M. , & Penninx, B. W. (2014). Major depressive disorder and accelerated cellular aging: Results from a large psychiatric cohort study. Molecular Psychiatry, 19(8), 895–901. 10.1038/mp.2013.151 [DOI] [PubMed] [Google Scholar]
- Wei, D. , Zhuang, K. , Ai, L. , Chen, Q. , Yang, W. , Liu, W. , … Qiu, J. (2018). Structural and functional brain scans from the cross‐sectional Southwest University adult lifespan dataset. Scientific Data, 5, 180134. 10.1038/sdata.2018.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner, P. , Scheel‐Krüger, J. , & Belzung, C. (2013). The neurobiology of depression and antidepressant action. Neuroscience and Biobehavioral Reviews, 37(10 Pt 1), 2331–2371. 10.1016/j.neubiorev.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Yüksel, D. , Engelen, J. , Schuster, V. , Dietsche, B. , Konrad, C. , Jansen, A. , … Krug, A. (2018). Longitudinal brain volume changes in major depressive disorder. Journal of Neural Transmission (Vienna), 125(10), 1433–1447. 10.1007/s00702-018-1919-8 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Liao, W. , Xu, Q. , Wei, W. , Zhou, H. J. , Sun, K. , … Lu, G. (2017). Hippocampus‐associated causal network of structural covariance measuring structural damage progression in temporal lobe epilepsy. Human Brain Mapping, 38(2), 753–766. 10.1002/hbm.23415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegel, E. R. (2010). The elements of statistical learning. Technometrics, 45(3), 267–268. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


