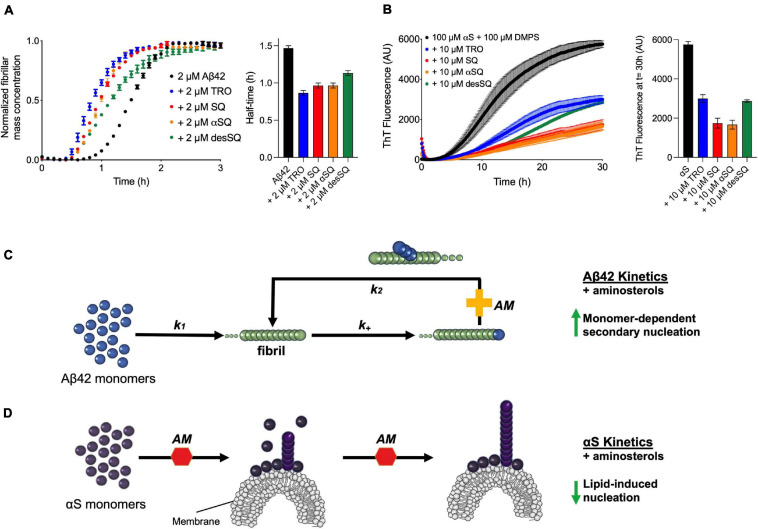
FIGURE 4.
Aminosterols modify the aggregation processes of Aβ42 and αS. (A) Kinetic profiles of the aggregation of 2 μM Aβ42 in the absence (black) or presence of an equimolar concentration of each aminosterol (represented with various colors). The bar plot shows the half-time of Aβ42 aggregation in the absence or presence of the aminosterols. (B) Kinetic profiles of the aggregation of 100 μM αS in the absence (black) or presence of 10 μM of each aminosterol (represented with various colors). Since trodusquemine has been characterized not to quench ThT or self-assemble into larger species under these conditions (Perni et al., 2018), the decrease in ThT signal for the aminosterols studied is not likely related to ThT artifacts or assemblies of TRO, such as micelles. The bar plot shows the ThT signal quantified after 30 h of αS aggregation in the absence or presence of the aminosterols. In panels (A–D), data represent mean ± s.e.m. of three technical replicates and are the kinetic traces reproduced from Figures 2, 3. (C) Schematic illustration of the effect of aminosterols (AM) on Aβ42 aggregation, where they enhance monomer-dependent secondary nucleation (k2). (D) Schematic illustration of the effect of aminosterols on αS aggregation, where they inhibit lipid-induced primary nucleation.