FIGURE 1.
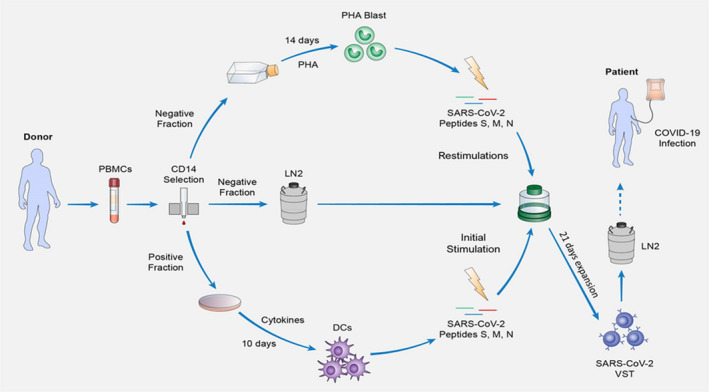
Expansion protocol. Schematic representation of the 31‐day expansion process of SARS‐CoV‐2 VST. The protocol starts with a density gradient separation of the blood sample donation to obtain peripheral mononuclear cells (PBMCs) that will be magnetically separated into CD14 positive and negative fractions. The CD14 positively selected monocytes are plated in petri dishes and stimulated with a cocktail of cytokines for a period of 10 days in order to differentiate them into DCs. From the CD14 negative fraction, a portion is cultivated in T75 flasks for 14 days in the presence phytohemagglutinin‐P (PHA) to induce lymphocyte blasts (PHA‐blasts), followed a 30 Gy gamma‐irradiation and cryopreservation until their usage as antigen presenting cells, while the rest of the negative fraction is cryopreserved until the start of the T cell culture. Once DCs are differentiated, this portion of the negative fraction is thawed and seeded on G‐Rex for an initial stimulation with SARS‐CoV‐2‐peptide loaded DCs (day 0), followed by 2 restimulations using peptide‐loaded PHA‐Blasts (day 7 and 14). On the last day of expansion (day 21), the culture is sampled for characterization and cells are harvested, aliquoted, cryopreserved, and stored in liquid nitrogen until their use for adoptive transfer into HLA‐matched severe COVID‐19 patients
