Abstract
Background
The outbreak of coronavirus disease 2019 (COVID‐19) rapidly spread across worldwide, posing a significant challenge to public health. Several shortcomings in the existing infectious disease management system were exposed during the pandemic, which hindered the control of the disease globally. To cope with this issue, we propose a window‐period framework to reveal the general rule of the progression of management of infectious diseases and to help with decision making at the early stage of epidemics with a focus on healthcare provisions.
Methods
The framework has two significant periods (dark‐window period and bright‐window period). Outbreak of COVID‐19 in China was used as an example for the application of the framework.
Results
The framework could reflect the progression of the epidemic objectively. The spread increased slowly in the dark‐window period, but rocketed up in the bright‐window period. The beginning of the bright‐window period was the time when healthcare personnel were exposed to a substantially high risk of nosocomial infection. Additionally, proper and prompt preventive actions during the dark‐window and bright‐window periods were substantially important to reduce the future spreading of the disease.
Conclusions
It was recommended that when possible healthcare provisions should upgrade to the highest level of alert for the control of an unknown epidemic in the dark‐window period, while countermeasures in the bright‐window period could be accordingly adjusted with full exploration and considerations. The framework may provide some insights into how to accelerate the control of future epidemics promptly and effectively.
What's known
The coronavirus disease 2019 (COVID‐19), has spread across worldwide, posing a significant challenge to public health. Several shortcomings in the existing emergency management system for infectious disease were exposed during the pandemic, especially at the early stage.
What's new
In this article, we proposed a framework to reveal the general rule of the progression of management of infectious diseases and to help with decision making of rapid and targeted countermeasures at the early stage of new epidemics.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19), caused by a novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2), has spread across China and many other countries worldwide, 1 posing a significant challenge to public health. For instance, as of 21st February 2021, the outbreak of COVID‐19 has yielded 111 763 898 confirmed cases and 2 455 331 deaths globally, including 101 700 cases and 4842 deaths in China, based on the data from World Health Organization. 2
In China, the occurrence of COVID‐19 cases dated back to early December 2019. 3 In retrospect, from December 2019 to early January 2020, more than 40 cases were reported. However, little was known about the pathogen and epidemiological characteristics of the disease at that moment; therefore, no prompt protective measures were taken in neither the majority of healthcare institutions nor communities by then. Since 16th January 2020, the number of daily confirmed cases started to rocket up, reaching the first peak on 4th February. Meanwhile, a large number of healthcare personnel were amongst the infected and/or suspected cases. Taken the 2‐to‐7‐day incubation period into consideration, 4 the peak of transmission of SARS‐CoV‐2 was accordingly dated back to late January in China.
Several shortcomings in the existing emergency management system for infectious disease were exposed during the pandemic, especially at the early stage. For example, defects included lack of rapid reaction to emerging infectious disease, failure to protect healthcare personnel, and insufficiency in medical resources in hospitals, to mention a few. The abovementioned issues hindered the control of the disease, not only in China, but in many other countries. To mitigate these issues, in this article, we propose a window‐period framework with two significant periods (dark‐window period and bright‐window period, both at the early stage of an unknown epidemic), taking the outbreak of COVID‐19 in China as an example. The term “window period” refers to the early stage of a new epidemic, while we cannot figure out the overall situation of the epidemic because of the lack of knowledge of the pathogen. Therefore we use the word “window” to reflect that our understanding would be largely restricted. The term “framework” indicates the structure of a system aiming to inform the decision making to control the epidemic of the disease. Our aim of proposing the framework is to reveal the general rule of the progression of management of infectious diseases and to help with decision making of rapid and targeted countermeasures at the early stage of new epidemics with a focus on healthcare provisions where, in general, the epidemic occurs initially, for the purpose of accelerating the control of future epidemics in a prompt and effective fashion.
2. METHOD
2.1. Development of the framework
The framework was developed based on two rounds of consensus discussions of a panel of experts who were specialised in infectious disease, epidemiology, evidence‐based medicine and hospital management. The framework we propose consists of four periods: pre‐window period, dark‐window period, bright‐window period and post‐window period. The start signs of each period are displayed in Figure 1. We define the occurrence of a first new case with unknown pathogens as the initiation indicator of pre‐window period. When there are two or more new cases that are epidemiologically and closely related to each other and with unknown pathogens appearing in the same hospital, the hospital is required to promptly document and report to the Chinese Centre for Disease Control and Prevention for further investigation. 5 This would place the authorities and hospitals on certain alert, albeit with unknown pathogens; therefore, it is considered as the start of dark‐window period. During the dark‐window period, it is the general practice to collect patients’ biological specimen for laboratory and aetiological investigation. Until the pathogen is clearly identified, all the hospitals and authorities remain to be blind to the new epidemic. Consequently, we define the date of identification of the pathogen as the start of the bright‐window period. Knowing the pathogen in the bright‐window period could enhance the targeted countermeasures performed, especially in the healthcare provisions. The date of relatively comprehensive understanding of the epidemiological characteristics of the pathogen is defined as the end of the bright‐window period, where the relatively comprehensive understanding refers to acquaintance with whether the disease is human‐to‐human transmissible, routes of transmission, and targeted disinfection methods, to name a few. Subsequently, the combat of infectious disease enters the post‐window period when pertinent policies can be carried out more widely and precisely.
FIGURE 1.

Proposed window‐period framework
Of note, if the pathogen identified at the start of the bright‐window period is found non‐novel and the disease can be under control with the existing services, the window‐period scheme will terminate automatically at the end of the dark‐window period and will be no longer applicable.
2.2. Data sources
Dates of the start sign of each period in the progress of COVID‐19 in China were obtained from documents and reports of the National Health Commission of the People's Republic of China, and Chinese Centre for Disease Control and Prevention. Daily number of confirmed and suspected cases for the overall population in China were collected from daily reports of the National Health Commission of the People's Republic of China and previous published literature. 3 , 6 The data were collected up to 11th February 2020, because the diagnostic criteria were substantially changed from 12th February on according to the Guidelines for the Diagnosis and Treatment of SARS‐CoV‐2 Infection (Trial Version 5). 7 In the Guidelines Version 5, patients with specific results from radiological examinations could be diagnosed as COVID‐19, regardless of their nucleic acid test results. Therefore, such a change in criteria made the data before and after 12th February not comparable. Data and figures on infected healthcare personnel were gathered from the Chinese Centre for Disease Control and Prevention. 8
2.3. Statistical analyses
Descriptive analyses were used to illustrate the trend of daily confirmed, suspected, cured and dead cases in each period. To assess the transmissibility of COVID‐19 in the framework, we conducted the analyses of time‐dependent reproduction number (Rt) using the “EpiEstim” and “CoarseDataTools” packages in R (version 3.6.0; R Development Core Team, Vienna, Austria). To calculate the Rt, we used the mean serial interval of COVID‐19 as of 7.5 days with a standard deviation of 3.4 days, based on a previously published model. 3
3. RESULTS
As Table 1 shows, during the progression of COVID‐19 in China, the start date of the bright‐window period was 8th January 2020 when the pathogen was identified as a novel coronavirus that was subsequently termed SARS‐CoV‐2. The post‐window period started on 27th January 2020, in which the Guidelines for the Diagnosis and Treatment of SARS‐CoV‐2 Infection (Trial Version 4) was published with a clear statement of high transmissibility of COVID‐19 and strong recommendations of strict regulations nationally. It thus turned out that the pre‐window period lasted for 10 days, dark‐window period 28 days and bright‐window period 19 days, respectively (Table 1).
TABLE 1.
Accumulative newly increased numbers of COVID‐19 cases for overall population stratified by the four periods in China
| Period | Dates | Confirmed | Suspected | Cured | Dead |
|---|---|---|---|---|---|
| Pre‐window period | 1st Dec 2019 ~ 10th Dec 2019 | 4 | 0 | 0 | 0 |
| Dark window period | 11th Dec 2019 ~ 7th Jan 2020 | 37 | 0 | 0 | 0 |
| Bright window period | 8th Jan 2020 ~ 26th Jan 2020 | 2703 | 6973 | 51 | 80 |
| Post‐window period (selected) a | 27th Jan 2020 to 11th Feb 2020 | 41 950 | 9094 | 4689 | 1033 |
For illustration, post‐window period is selected up to 11th Feb 2020; the period is still ongoing.
Accumulative numbers of newly confirmed, suspected, cured and dead cases for the overall population stratified by the four periods in China are presented in Table 1. The daily confirmed cases increased slowly in the per‐ and dark‐window period, while there were 37 new cases confirmed during the dark‐window period. Subsequently, the number started to soar during the bright‐window period (Figure 2A), yielding a newly confirmed amount of 2,703 cases at the end of this period. There were 3,887 newly confirmed cases reported on the eighth day in the post‐window period (4th February 2020; Figure 2A), which reached the spike during the window‐period framework. The peak of Rt was 48.25 at the middle of the bright‐window period (16th January, Figure 3), indicating substantially strong transmissibility of COVID‐19 at the early stage. second peak of Rt was 6.55 on 24th January.
FIGURE 2.

Daily increased numbers of COVID‐19 cases for overall population and healthcare personnel in China (note–A, The number of diagnosed patients quickly increased in the bright‐window period. Eight days after the window‐period ends, the confirmed case started to decrease; B, Peak of diagnosis of healthcare personnel was in the middle of bright‐window periods. 8 Considering the incubation period of 2‐7 days, they were supposed to be infected at the beginning of the bright‐window period.)
FIGURE 3.
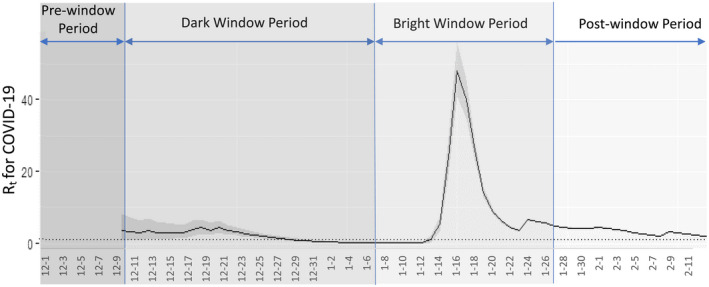
Time‐dependent reproduction number (Rt) stratified by the four periods for COVID‐19 in China
Regarding the infection of healthcare personnel because of COVID‐19, Figure 2B, 8 presents the daily increased cases, where the peak of newly confirmed cases laid in between the middle (20th January) and late (27th January) during the bright‐window period. Considering the incubation period of 2 to 7 days, the peak of infected healthcare personnel appeared at the early stage of bright‐window period. When compared with the daily increased cases for the overall population (Figure 2A), the peak of infected cases was approximately 8 days earlier for the healthcare personnel.
4. DISCUSSION
We proposed a framework to help understand the process of emerging infectious disease, aiming to provide some insight into future scientific decision making of rapid and targeted countermeasures at the early stage of new epidemics. In our retrospective analyses of the COVID‐19 epidemic in China, we found that the spread increased slowly in the dark‐window period, but rocketed up in the bright‐window period. Eight days after the end of the bright‐window period, the daily number of confirmed cases started to decrease for the overall population. These results indicated that the positive trend in the control of COVID‐19 appeared shortly after the countermeasures that were taken place in the bright‐window period. Moreover, our findings reflected that the beginning of the bright‐window period was the time when healthcare personnel were exposed to a substantially high risk of nosocomial infection, where the transmission amongst healthcare personnel might serve as an important predictive indicator of the subsequent outbreak in the overall population. Taken together, our results suggested that the proposed window‐period framework could reflect the general rule of progression of a new epidemic, and proper and prompt preventive actions during the dark‐window and bright‐window periods were significantly important to reduce the future spread of the epidemic.
There were several studies directly or indirectly supporting our framework. Some studies implied that the improvement in the combat of the COVID‐19 outbreak could be because of the interventions that were implemented during bright‐window period. First, a similar study analysed the COVID‐19 outbreak in Wuhan by five periods according to the key events and interventions 9 ; it was concluded that multifaceted public health interventions were temporally related with improved control of the COVID‐19 outbreak in Wuhan. Several studies have reported the positive effect of Wuhan lockdown. 10 , 11 , 12 , 13 For example, without the Wuhan lockdown or the national emergency response, the confirmed cases outside Wuhan by 19th February would have increased from 29 839 to 744 000 (standard deviation: 156 000). 13 Second, isolation of all suspected and diagnosed cases at assembly sites was another important measure. One study suggested that home‐based isolation may fail to effectively prevent both household and non‐household transmissions of COVID‐19. 14 Furthermore, prompt and rigorous isolation was linked to a reduction in the effective reproduction number of COVID‐19. 14 , 15 These findings highlighted the significance of the rapid establishment of assembly sites. 16 To achieve this, Fangcang shelter hospitals could be one of the optimal options because of their rapid constructions, massive scales and low costs. 17 Even though Fangcang hospitals were built in the post‐window period in Wuhan, they may own the potential to be used in the early stage of epidemic. Third, travel restrictions, especially the restriction on air travel were substantially helpful to prevent imported cases. 18 , 19 , 20 Nevertheless, different from the aforementioned studies, our window‐period framework was established to reflect the general rules emerging infectious diseases stratified by different stages during their progression, with illustrations that were combined with the nature of disease (incubation period and Rt), newly confirmed case numbers, and significant events.
Moreover, apart from the results and implications for the overall population, our framework had a special focus on infections of healthcare personnel who provided care for patients and ensured preventive measures were fully implemented in healthcare provisions. Transmission amongst healthcare personnel, therefore, resulted in the shortage of workforce and inability to serve and admit patients, making the combat to bring the outbreak under control more challenging and extremely difficult. As of April 2020, there had been 22 073 healthcare personness who were infected with COVID‐19 in 52 countries. 21 According to the data in China (Figure 2B), healthcare personnel were exposed to a substantially high risk of nosocomial infection especially at the beginning of bright‐window period. This could be because of the lack of knowledge of the epidemiological characteristics of the pandemic, undue policies and decisions made in healthcare provisions, insufficient protective equipment and self‐protection awareness. Since 24th January, over 40 000 healthcare personnel from other provinces of China had been dispatched to Hubei Province in succession to support the combat of COVID‐19. With the experience and lessons learnt from the early bright‐window period and especially with the stringent regulations and policies fully enforced in all healthcare provisions, there was no nosocomial infection reported in those external healthcare personnels who supported Hubei Province. All these findings highlighted the importance of extreme caution and prompt precautions in healthcare provisions, especially at the beginning of the bright‐window period with the specific pathogen identified.
In the dark‐window period as another significant stage of an unknown epidemic, information on the infected cases are usually ambiguous, sparse and with unexplained reasons; therefore, the severity of the emerging infectious disease is generally underestimated. Nevertheless, even though the healthcare resources appear sufficient given the small number of infected cases, healthcare provisions are in extreme danger because they are blind to the new epidemic. We, therefore, recommend when possible, healthcare provisions should upgrade to the highest level of alert for the control of an unknown epidemic. These reactions and countermeasures may face a dilemma of balancing potential public panic and waste of healthcare resources, and controlling nosocomial transmission; however, with the astonishing number of infected healthcare providers and the global spread in the overall population, implementing strict regulations in healthcare provisions in dark‐window period may probably be a practical and cost‐effective approach to fighting an unknown epidemic. For example, the Department of Respiration of a hospital in Wuhan started the highest protective countermeasures during the dark‐window period of the COVID‐19 outbreak. Consequently, none of their healthcare personnel had been infected as of 1st June 2020, even though they had served hundreds of COVID‐19 patients cumulatively. 22 Furthermore, with the development of aetiology and the advances in laboratory technologies, the duration of the dark‐window period has been significantly shortened in the recent decades. For instance, SARS, Middle East Respiratory Syndrome (MERS) and COVID‐19 were the three emerging coronaviruses diseases that occurred in 2003, 2012 and 2019. The dark‐window period of them lasted for approximately 5 months, 3 months and 1 month, respectively. Besides, some “emerging infectious disease” would be eventually found to be caused by existing pathogens that have been widely investigated. Similarly, with the advances in medicine, the dark‐window period would sustain a shorter time; for instance, the mean interval between the onset and diagnosis of pneumonia of unknown aetiology was reported to be only 6 days. 23 Therefore, the duration of highest level alert in the dark‐window period would be further shortened as expected, for both the emerging disease with a non‐novel pathogen found subsequently and the true epidemic caused by a novel pathogen. The short duration of the dark‐window period may, at least in part, justify the upgraded level of alert in healthcare provisions.
In the bright‐window period with the pathogen identified, countermeasures could be accordingly terminated or adjusted based on our knowledge of previous familiar pathogens with caution. Nonetheless, undue adjustments because of underestimation of the new pathogen and insufficient understanding of its epidemiological characteristics would yield suboptimal control of nosocomial and community infection; therefore adjusted regulations in the bright‐window period require full exploration, discussion and considerations. Nevertheless, as data are shown for infected healthcare providers in Wuhan and outside Hubei, as well as the peak of Rt appeared at the middle of the bright‐window period, again prompt and strict policies and regulations in healthcare provisions especially at the beginning of the bright‐window period would play a critical role in controlling nosocomial infection.
In recent decades, there have been several novel infectious diseases appearing globally including SARS, MERS, Influenza A virus subtype H1N1 and the current COVID‐19. There is no optimal protocol to cope with these emerging diseases yet, especially at their early stage. Our study proposed a novel window‐period framework to highlight the importance of early stages of new epidemics, which may provide some insights into decision aids for both healthcare provisions and authorities. However, the data used for illustrations were suboptimal. For instance, the number of confirmed or suspected cases at the very beginning of the pandemic could be under‐reported because of the inability to identify all patients when they surged to healthcare provisions. The incubation period of COVID‐19 varied between 2 and 7 days, yielding the trend of daily confirmed cases not able to exactly reflect the actual transmission. Furthermore, we could not carry out stratified analysis for each province or geographical area of China because of the unavailability of the relevant data. China is highly diverse in economy, culture and geographical conditions; therefore, further studies are required to analyse and compare data amongst different areas of China, aiming to improve our proposed framework for targeted and tailed protocols for different regions. Moreover, various regulations and policies for reporting new cases with unknown pathogens in different countries or regions may yield different start points and thus lengths of dark‐window period, which may compromise the generalisability and comparability of applying the framework to different areas.
In conclusion, we proposed a window‐period framework to reveal the general rule of the progression of infectious diseases and to help with decision making of rapid and targeted countermeasures at early stage of new epidemics, with a focus on healthcare provisions. It was recommended that when possible healthcare provisions should upgrade the level of alert for the control of an unknown epidemic in the dark‐window period, while countermeasures in the bright‐window period could be accordingly adjusted with full exploration and considerations. The framework may provide some insights into how to accelerate the control of future epidemics in a prompt and effective fashion.
DISCLOSURES
None.
Li Z, Li C, Wu X, Li G, Li G, Tian J. Development and application of a new framework for infectious disease management at the early stage of new epidemics: Taking COVID‐19 outbreak in China as an example. Int J Clin Pract. 2021;75:e14174. 10.1111/ijcp.14174
Ziyi Li and Cheng Li contributed equally to this work.
Funding information
This work was supported by the Science Foundation of Guangdong Second Provincial General Hospital (Grant recipient: Dr Guowei Li; Grant no. :YY2018‐002) and Doctoral Workstation Foundation of Guangdong Second Provincial General Hospital (Grant recipient: Dr Ziyi Li; Grant no. :2019BSGZ027)
Contributor Information
Guowei Li, Email: lig28@mcmaster.ca.
Junzhang Tian, Email: zhanggd2h@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med. 2020;382:727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . COVID‐19 Weekly Epidemiological Update. 2021. [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Health Commission of the People’s Republic of China . National Scheme for Surveillance, Investigation and Management of Unexplained Pneumonia Cases. 2007.
- 6. National Health Commission of the People's Republic of China . 2020. [DOI] [PMC free article] [PubMed]
- 7. National Health and Health Commission of the People's Republic of China . Guidelines for the Diagnosis and Treatment of Novel Coronavirus. (2019‐nCoV) Infection by the National Health Commission (Trial Version 8). 2020.
- 8. Epidemiology Working Group for NCIP Epidemic Response . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Chin J Epidemiol. 2020;41:145‐151. [Google Scholar]
- 9. Pan AN, Liu LI, Wang C, et al. Association of Public Health Interventions With the Epidemiology of the COVID‐19 Outbreak in Wuhan, China. JAMA. 2020;323:1915. 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lau H, Khosrawipour V, Kocbach P, et al. The positive impact of lockdown in Wuhan on containing the COVID‐19 outbreak in China. J Travel Med. 2020;27:taaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhong P, Guo S, Chen T. Correlation between travellers departing from Wuhan before the Spring Festival and subsequent spread of COVID‐19 to all provinces in China. J Travel Med. 2020;27:taaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bogoch II, Watts A, Thomas‐Bachli A, et al. Potential for global spread of a novel coronavirus from China. J Travel Med. 2020;27:taaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tian H, Liu Y, Li Y, et al. An investigation of transmission control measures during the first 50 days of the COVID‐19 epidemic in China. Science. 2020;368:638‐642. 10.1126/science.abb6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee EC, Wada NI, Grabowski MK, Gurley ES, Lessler J. The engines of SARS‐CoV‐2 spread. Science (New York, N.Y.). 2020;370:406‐407. [DOI] [PubMed] [Google Scholar]
- 15. Wilasang C, Sararat C, Jitsuk NC, et al. Reduction in effective reproduction number of COVID‐19 is higher in countries employing active case detection with prompt isolation. J Travel Med. 2020;27:taaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickens BL, Koo JR, Wilder‐Smith A, Cook AR. Institutional, not home‐based, isolation could contain the COVID‐19 outbreak. Lancet (London, England). 2020;395:1541‐1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet (London, England). 2020;395:1305‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dickens BL, Koo JR, Lim JT, et al. Strategies at points of entry to reduce importation risk of COVID‐19 cases and reopen travel. J Travel Med. 2020;27:taaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costantino V, Heslop DJ, MacIntyre CR. The effectiveness of full and partial travel bans against COVID‐19 spread in Australia for travellers from China during and after the epidemic peak in China. J Travel Med. 2020;27:taaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuite AR, Bhatia D, Moineddin R, et al. Global trends in air travel: implications for connectivity and resilience to infectious disease threats. J Travel Med. 2020;27:taaa070. [DOI] [PubMed] [Google Scholar]
- 21. National Health Commission of the People’s Republic of China . Daily Reports of COVID‐19. Vol. 2020. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Ye H, Chen W. Zero Infection in the Department Lead by Him. Vol. 2020 (Chagjiang Net); 2020. [Google Scholar]
- 23. Wang Y. Evaluation of the Surveillance System for pneumonia of unknown etiology: Chinese center for disease control and Prevention. 2017;26.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


