Figure 1.
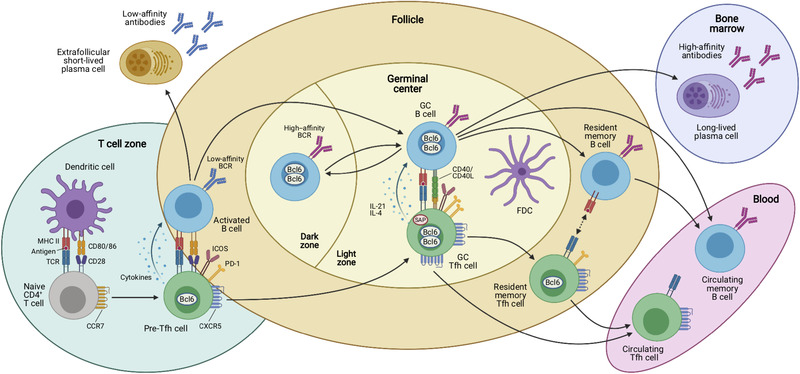
Antigen‐dependent multistep differentiation of T follicular helper cells. Naïve CD4+ T cells are primed by dendritic cells (DCs) in the T‐cell zones of secondary lymphoid organs such as the spleen or lymph nodes. This antigen‐specific interaction is mediated by presentation of processed peptides by MHC‐II molecules that are recognized by the cognate TCR. Together with the expression of costimulatory molecules and production of cytokines, these signals induce the Tfh cell differentiation program that is characterized by upregulation of the chemokine receptor CXCR5 and downregulation of CCR7, which allows these cells to migrate to the T/B border where they interact with activated B cells. This second interaction with an APC type coincides with the expression of characteristic costimulatory (e.g. ICOS) and coinhibitory receptors (e.g. PD‐1) by the Tfh cells, which may be called “pre‐Tfh” cells at this stage. After this cellular interaction, some activated B cells become extrafollicular short‐lived plasma cells (PCs) that secrete low‐affinity antibodies and some of the interacting T and B cells relocate to the follicle to form germinal centers (GCs). In these highly specialized microanatomical structures that consist of dark zones (DZ) and light zones (LZ), GC Tfh and B cells continue to interact in an antigen‐specific manner. GC Tfh cells are more polarized and express higher levels of PD‐1, Bcl6, and CXCR5 than “pre‐Tfh” cells. In addition, they express the adaptor molecule SAP that is important for interactions with GC B cells and they produce IL‐21 and IL‐4 that act on the B cells. While GC B cells mutate their immunoglobulin genes and proliferate in the DZ, GC Tfh cells provide essential signals to B cells in the LZ of the GC, where they cooperate with follicular dendritic cells (FDCs) in the selection of high‐affinity B‐cell clones and in the generation of memory B cells, which recirculate in the blood, and long‐lived plasma cells that find their niche in the bone marrow to maintain serological memory through the secretion of high‐affinity antibodies. Once the GC reaction is resolved, some memory CXCR5+ CD4+ T cells reside in close proximity with memory B cells in lymphoid organs, while others are released from the draining lymphoid organs and circulate in the blood. Both memory Tfh cell subsets express decreasing amounts of Bcl6, CXCR5, and other costimulatory/inhibitory molecules as compared to their effector counterparts. Upon antigen rechallenge, these cells rapidly acquire the effector functions of Tfh cells and strongly support secondary immune response.
