Abstract
Purpose
To perform a retrospective review of Coronary Artery Disease Reporting and Data System (CAD-RADS) adoption at a high-volume cardiac CT service.
Materials and Methods
In this retrospective study, the adoption of CAD-RADS in 6562 coronary CT angiography (CTA) reports from January 1, 2017, to February 13, 2020, was evaluated. Reports without CAD-RADS were classified as opt-outs or exceptions to CAD-RADS. CAD-RADS classifications were retrospectively assigned to the opt-outs and the clinical indications for coronary CTA.
Results
CAD-RADS scores were reported in 95% (6264 of 6562) of cases. Among the 5% (n = 298) of reports not reported according to CAD-RADS, 58% (n = 172) were considered opt-outs and 42% (n = 126) were exceptions. Cases with higher degree of stenosis, stents, and coronary artery bypass grafts (CABGs) occurred more often in opt-outs versus reports with CAD-RADS (odds ratio [OR], 8.3 [95% CI: 1.6, 42.1]; P < .001). The quarterly opt-out rate decreased over consecutive quarters in the 1st year (OR, 0.77 [95% CI: 0.61, 0.96]; P = .01), then stabilized. Quarterly opt-out rate for patients with stents decreased over time (OR, 0.82 [95% CI: 0.73, 0.92]; P = .008), as did the opt-out rates in patients with CABG (OR, 0.83 [95% CI: 0.76, 0.91]; P < .001). Exceptions (n = 126) included coronary dissections (44%), anomalous coronary arteries (41%), coronary artery aneurysms or pseudoaneurysms (10%), vasculitis (2%), stent complications (2%), and extrinsic compression of grafts (2%).
Conclusion
CAD-RADS was adopted rapidly and widely. Readers opted out of its use most often in complex cases of CAD, and the most common exceptions were coronary dissections and anomalous coronary artery.
Keywords: Coronary Arteries, CT Angiography
© RSNA, 2021
Keywords: Coronary Arteries, CT Angiography
Summary
At the authors’ institution, Coronary Artery Disease Reporting and Data System (CAD-RADS) was adopted rapidly and widely with an adoption rate in 95% of coronary CT angiographic examinations over a 3-year period. The most common exceptions were coronary dissections and anomalous coronary arteries; readers opted out of CAD-RADS most often in complex CAD, but the rate of opt-outs decreased over time.
Introduction
In 2016, radiology and cardiology societies jointly introduced the Coronary Artery Disease Reporting and Data System (CAD-RADS), a standardized classification of coronary artery stenosis in coronary CT angiography (CTA) focused on CAD (1). CAD-RADS comprises two parts: classifiers and modifiers. Classifiers correspond to the percentage of diameter stenosis of the most severe anatomic coronary obstruction which is graded from 0 to 5 (none to total occlusion). Every segment measuring more than 1.5 mm in diameter should be assessed, but only the clinically most relevant stenosis is considered for the final classification. If CAD-RADS is equal to or less than 3 and there are nondiagnostic segments, the category N should be applied. Modifiers are other descriptors of coronary artery features found by coronary CTA. They are nonassessable arterial segments (N), stents (S), coronary artery bypass grafts (CABGs; represented as G), and vulnerable plaques (V). Consensus suggests further cardiac workup and management for stable or acute chest pain according to the final CAD-RADS classifier (1).
Since its introduction, there have been 50 studies indexed in PubMed with the term “CAD-RADS” included in the abstract or in the title (Fig 1). CAD-RADS has been shown to accurately predict major adverse cardiovascular events, defined as unstable angina, myocardial infarction, or death, in patients with stable chest pain, with performance equal to or higher than other traditional scores, such as coronary artery calcium score and the previous Society of Cardiovascular Computed Tomography coronary stenosis scoring system (2–4). CAD-RADS also has been shown to correlate with the degree of stenosis measured by invasive coronary angiography with high sensitivity (100%), specificity (96.8%–98.7%), and accuracy (98.3%–99.3%) (5). Overall, available research suggests that CAD-RADS offers a clinically useful and appropriate categorization of CAD.
Figure 1:
Timeline plots of total quarterly PubMed citations resulting from the search “CAD-RADS”[Title/Abstract] OR “CADRADS”[Title/Abstract]. The date of the search was January 25, 2021.
CAD-RADS, however, was not intended to be applied to nonatherosclerotic causes of obstruction, designated “exceptions” in this study. Exceptions are far less frequent than atherosclerotic causes of obstruction but remain important differential diagnostic considerations and are increasingly recognized in recent years (for example, coronary dissection). Patterns of CAD-RADS adoption and rates of exceptions have not been quantitatively evaluated in the literature. In this work, we evaluate the adoption of CAD-RADS, the frequency of exceptions, and the frequency of and reasons behind opt-out cases (ie, cases in which CAD-RADS should have been, but was not applied).
Materials and Methods
Inclusion and Exclusion Criteria
This retrospective study was performed under an institutional review board–approved and Health Insurance Portability and Accountability Act–compliant protocol. Requirement for informed consent was waived. As CAD-RADS was formally implemented at our institution in the fourth quarter of 2016, all coronary CTA reports from January 1, 2017, to February 13, 2020, were considered for retrospective analysis. Our service is a joint effort of the department of radiology and division of cardiology at our hospital, with staff readers and trainees from both specialties working jointly, with final reports only by staff imagers; all staff have completed dedicated subspecialty fellowship training in cardiac CT. Corresponding reports were assessed and classified by two cardiac imaging research fellows (A.K.T. and V.T.) and one cardiovascular imaging clinical fellow (R.J.G.), who had 1, 8, and 3 years of experience, respectively, in cardiovascular research. They were supervised by a cardiovascular imaging staff member with more than 10 years post–subspecialty fellowship experience (B.G.). Noncoronary cardiac CT examinations, those performed at an outside institution and secondarily interpreted at our hospital, and studies performed for reasons other than coronary artery assessment (calcium scoring, valve evaluation, surgical planning, research, cardiac masses, pulmonary and cardiac veins, and noncoronary congenital heart disease) were excluded (Fig 2).
Figure 2:
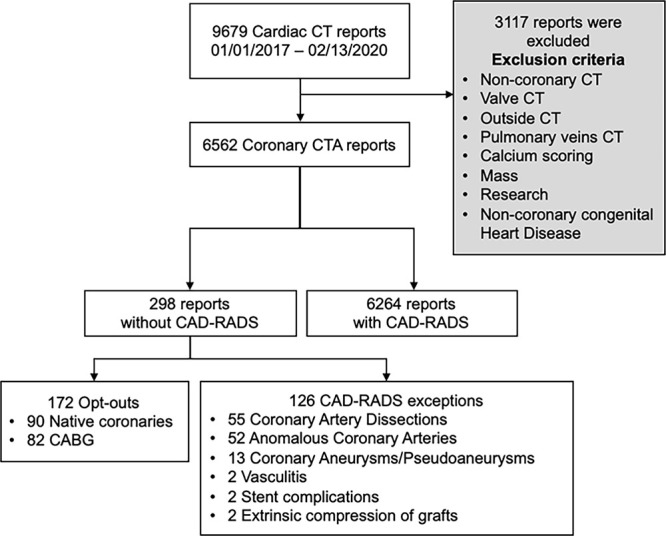
Study flow diagram of coronary CTA examinations considered for analysis. CABG = coronary artery bypass graft, CAD-RADS = Coronary Artery Disease Reporting and Data System, CTA = CT angiography.
Analysis of Reports
Reports meeting the eligibility criteria without a CAD-RADS classification in the impression were classified as follows:
-
Opt-out: stenosis was not graded using CAD-RADS despite CAD being the primary differential diagnostic consideration
Opt-out without prior CABG
Opt-out with prior CABG
-
Exceptions: nonatherosclerotic or nonstenotic disease(s) were at the top of the differential diagnosis
Coronary artery dissection
Anomalous coronary artery
Vasculitis
Coronary artery aneurysm
Coronary artery pseudoaneurysm
Stent complications (eg, fracture)
Extrinsic compression of graft
We analyzed age, sex, and status (acute vs stable presentation) of reports with CAD-RADS and opt-outs. We retrospectively assigned a CAD-RADS score to each of the opt-out cases and evaluated their clinical indication for the coronary CTA.
Statistics
The primary analysis variables for this study were rates of opt-out reports, retrospectively applied CAD-RADS scores for opt-out examinations, reason for opt out, exceptions, and reported CAD-RADS scores. R.J.G. performed statistics with generalized linear modeling in SPSS 26 (IBM); statistical significance was taken as a two-sided P value less than .05.
Results
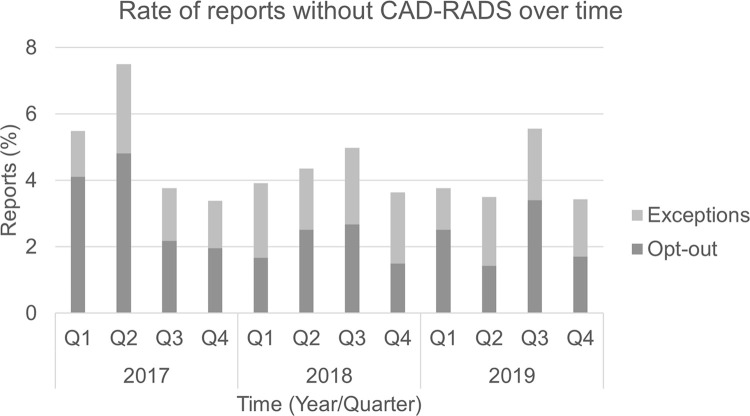
CAD-RADS scores were given in 95% (6264 of 6562) of eligible coronary CTA reports. Among the 5% (298 of 6562) of reports not listing a CAD-RADS score, 58% (172 of 298) were opt-outs and 42% (126 of 298) were exceptions. The quarterly rate of opt-out CTA examinations decreased over time (odds ratio [OR], 0.94 [95% CI: 0.90, 0.99]; P = .02), this average rate driven by decreasing opt-out rates in the 1st year (OR, 0.77 [95% CI: 0.61, 0.96]; P = .01), which stabilized in subsequent years (OR, 0.98 [95% CI: 0.72, 1.32]; P = .87). Quarterly opt-out rate for patients with stents decreased over time (OR, 0.82 [95% CI: 0.73, 0.92]; P < .001), as did the opt-out rates in patients with CABG (OR, 0.83 [95% CI: 0.76, 0.91]; P < .001). The overall rate of exceptions did not vary over time (OR, 1.0 [95% CI: 0.95, 1.05]; P = .93) (Fig 3).
Figure 3:
Rate of reports without Coronary Artery Disease Reporting and Data System (CAD-RADS) over time.
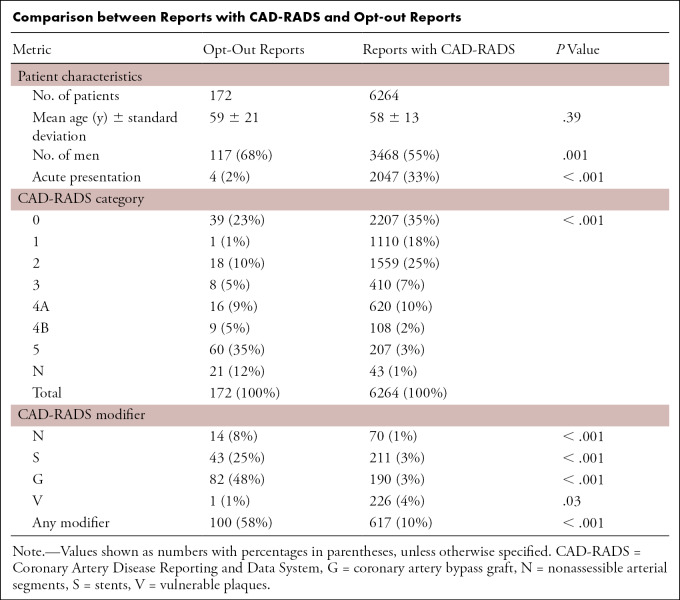
There was no difference in age between patients with CAD-RADS reported and opt-outs (58 years ± 13 vs 60 years ± 21; P = .35) (Table). The higher proportion of men in the opt-out group (68%) compared with those with CAD-RADS scores (55%) was significant (P = .001) (Table); however, this could be attributed to the higher prevalence of stents and CABG in men, as this relationship was not detected when adjusted for sex (OR, 0.98 [95% CI: 0.7, 1.4]; P = .91). A total of 2% (four of 172) of the opt-outs and 32% (2047 of 6462) of the reports with CAD-RADS were in the setting of acute presentation (P < .001).
Comparison between Reports with CAD-RADS and Opt-out Reports
Opt-outs in general had higher degree of stenosis compared with CAD-RADS reports (group-wide OR of greater stenosis, 8.3 [95% CI: 1.6, 42.1]; P < .001). This difference is due to the high opt-out rate of CTA findings with total occlusions; when total occlusion cases were excluded, there was no significant tendency toward greater stenosis (OR, 1.6 [95% CI: 0.38, 6.31]; P = .12). Stents were present in 25% (43 of 172) of the opt-out reports compared with 3% (211 of 6264) of CAD-RADS reports (P < .001). CABG was also overrepresented in opt-out reports, 48% (82 of 172) compared with 3% (190 of 6264) in CAD-RADS reports (P < .001) (Table). The distribution of vulnerable plaques between the two groups was similar (1% [one of 172] of the opt-out reports and 4% [226 of 6264] of CAD-RADS reports; P = .03). Nondiagnostic studies and nondiagnostic segments were more frequent in the opt-outs than in reports with CAD-RADS (12% [21 of 172] vs 1% [43 of 6264] and 8% [14 of 172] vs 1% [70 of 6264], respectively; P < .001 for both). All 14 opt-out cases with nondiagnostic segments were CAD-RADS 4A, 4B, or 5 cases. In comparison, 50% (35 of 70) of the reports with CAD-RADS with nondiagnostic segments were CAD-RADS 4A, 4B, or 5 cases.
In the opt-out native coronaries group (90 of 172 opt-outs), 60% (54 of 90) of the coronary CTA examinations were requested to evaluate CAD burden, and 22% (20 of 90) were requested to rule out anomalous coronary artery (but upon imaging, an anomaly was not found). A total of 54% (19 of 35) of the opt-out native coronaries with CAD-RADS 0 were requested to rule out anomalous coronary artery. In the opt-out CABG group, 71% (58 of 82) of the studies were requested for CAD evaluation, followed by 17% (14 of 82) for percutaneous coronary intervention or CABG planning. Other clinical indications were evaluation of aorta, preoperative evaluation, postsurgical follow-up, percutaneous coronary intervention or CABG planning, intracardiac thrombus, rule out coronary aneurysm, rule out coronary dissection, and vascular abnormalities.
Most notable were the 126 appropriate CAD-RADS exceptions. Among the exceptions, 44% (55 of 126) were reported as likely (or known) spontaneous coronary dissections, 41% (52 of 126) had an anomalous coronary artery, 10% (13 of 126) were coronary artery aneurysms or pseudoaneurysms, 2% (two of 126) were likely vasculitis, 2% (two of 126) were stent complications, and 2% (two of 126) were extrinsic compression of grafts.
Discussion
Overall, CAD-RADS scores were given in the majority of reports. Even with an initial adoption rate of 95%, the rate of reports without CAD-RADS scores decreased during the 1st year and was driven by a decreased opt-out rate. Opt-out cases composed the majority of the reports without CAD-RADS in our data set. Those reports tended to describe greater degrees of stenosis and had higher rates of prior stents or CABG than reports that included CAD-RADS. Vulnerable plaques were infrequent in both opt-outs and reports with CAD-RADS. Our findings suggest that advanced CAD and previous percutaneous coronary intervention or CABG may have affected the decision of not applying CAD-RADS. While this is in keeping with CAD-RADS guidelines emphasizing the sensitivity and negative predictive value of coronary CTA (maximizing clinical value in patients with acute or stable chest pain and no prior diagnosis of CAD [1]), it suggests a potential need to educate new users of CAD-RADS, and perhaps an avenue to improve future versions of CAD-RADS to account more readily for more complex disease.
The presence of more nondiagnostic segments or studies in the opt-outs suggests that those cases were technically more difficult, which is reinforced by the higher number of advanced cases of atherosclerosis in this group. The value of CAD-RADS in complex disease or patients with known CAD has been increasingly appreciated by our readers and referring physicians, as reflected in our data showing progressively increased use of CAD-RADS in the setting of advanced CAD, CABG, and stents. Coronary CTA is an appropriate tool for CABG evaluation (6,7), but anatomy is more complex. On the basis of lower adoption rates early in CAD-RADS implementation by our readers, we suspect that these more complex situations can be harder to apply, especially for trainees and staff new to the CAD-RADS system.
The clinical setting and indication for imaging may also have influenced the use of CAD-RADS. The readers tended to use CAD-RADS more frequently in the setting of acute presentation. In 22% of the opt-out reports of native coronaries, the examination was requested to rule out coronary artery anomalies (and revealed no anomaly nor plaque), and 100% could thus retrospectively be classified as CAD-RADS 0. In those cases, the report answered the provider’s primary clinical question by ruling out anomalous coronary artery and reporting no evidence of CAD. In 17% of the opt-out reports with prior CABG, the examination was ordered for percutaneous coronary intervention or CABG planning. In those cases, we speculate that the reader may have opted out due to the fact that a treatment plan was already in place, and CAD-RADS recommendations would not change management, especially in nuanced situations arising in more complex disease.
CAD-RADS exception rate was constant over time after an initial stabilization period. Coronary dissections and anomalous coronary arteries were the most common causes of exceptions and are potentially serious nonatherosclerotic etiologies of coronary obstruction. While coronary artery anomalies are rare, with a reported incidence between 0.2% and 2.3% (8,9), coronary CTA is the modality of choice for optimal assessment (10). Similarly, the prevalence of coronary dissection is thought to be 1%–4% and may be higher in younger, female, peripartum patients with or without underlying atherosclerotic disease (11); the clinical presentation of coronary dissections are increasingly recognized, and CT can be used for follow-up of established dissections and occasionally (in stable patients) for initial diagnosis. Other exceptions were related to pseudoaneurysm, aneurysm, vasculitis, stent fractures, and external compression of grafts; these are differential considerations for chest pain and can occur superimposed with CAD but are excluded from CAD-RADS guidelines. As a solution to this problem, some radiologists used CAD-RADS score followed by the term “equivalent” (eg, “CAD-RADS 2 equivalent” for a patient with anomalous coronary artery and concomitant mild nonobstructive atherosclerosis in the nonanomalous segments). In the era of high-sensitivity troponin, the role of coronary CTA in acute coronary syndrome workup may evolve (12), and there may be an increased number of exceptions on the basis of management recommendations. CAD-RADS recommendations could be updated to follow this new reality (the 2016 version specifies only for management after an initial negative traditional serum troponin assay leads to coronary CTA). Notably, our readers and physicians alike have found the primary value of CAD-RADS in affirming management in patients with no disease or nonobstructive disease (ie, reassurance that usually no further testing is necessary in CAD-RADS 0–2 cases); higher degrees of stenosis and disease more often generate situations that are understandably more difficult to group into prespecified management pathways.
This study had limitations. It evaluated the adoption of CAD-RADS in a single academic quaternary hospital with a relatively high number of complex patients and rare cases. Moreover, our service consists of a mixture of trainees and more experienced cardiovascular imaging staff (staff and trainees are composed of both cardiologists and radiologists, with a slight predominance of radiologists during the study period). Our referral pattern may not correspond to trends found in other institutions, although in prior studies we have found remarkably similar clinical referral patterns when compared to other clinical data in research sites worldwide when comparing specific populations, such as patients receiving emergency chest pain workup (13). As the data were collected retrospectively, the reason for opt-out reports was not systematically prospectively documented in this study.
On the basis of our findings, we speculate that a modifier for CAD-RADS exceptions could be created so that stenoses of other etiologies can still be graded using CAD-RADS. This modifier would have three purposes: it would make possible the evaluation of CAD in cases of CAD-RADS exceptions, it would provide a framework for research into those etiologies, and it would indicate to the provider that CAD-RADS recommendations pertinent to CAD would not apply. Management decisions would still be made on an individual basis, using clinical data and other detailed information in the report.
CAD-RADS was rapidly and widely applied to coronary CTA reports in our institution after its implementation. Opt-outs (ie, scenarios where CAD-RADS was intentionally not used) included complex situations such as prior stents, CABG, or advanced CAD, but we observed a reduction of these opt-out cases over time. The most common clinical indications resulting in exceptions to the 2016 CAD-RADS grading system included anomalous coronary artery, coronary artery dissection, coronary aneurysm, or pseudoaneurysm.
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: A.K.T. disclosed no relevant relationships. V.T. disclosed no relevant relationships. R.J.G. disclosed no relevant relationships. J.D. disclosed no relevant relationships. S. Gupta disclosed no relevant relationships. S. Garrana Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author receives author royalties from Elsevier, unrelated to this study. Other relationships: disclosed no relevant relationships. V.K. disclosed no relevant relationships. A.T.R. disclosed no relevant relationships. M.T.L. disclosed no relevant relationships. N.M. disclosed no relevant relationships. U.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received consultancy fees from Recor and Duke University; author’s institution has grants/grants pending from KOWA, Astra Zeneca, Medimmune, and HeartFlow. Other relationships: disclosed no relevant relationships. S.H. disclosed no relevant relationships. B.G. Activities related to the present article: author’s institution has grant support from Siemens Healthineers for cardiac CT research unrelated to this work; author is on the editorial board of Radiology: Cardiothoracic Imaging (not involved in handling of the article). Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CABG
- coronary artery bypass graft
- CAD-RADS
- Coronary Artery Disease Reporting and Data System
- CTA
- CT angiography
- OR
- odds ratio
References
- 1.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016; 10(4):269–281. [DOI] [PubMed] [Google Scholar]
- 2.Bittner DO, Mayrhofer T, Budoff M, et al. Prognostic Value of Coronary CTA in Stable Chest Pain: CAD-RADS, CAC, and Cardiovascular Events in PROMISE. JACC Cardiovasc Imaging 2020; 13(7):1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie JX, Cury RC, Leipsic J, et al. The Coronary Artery Disease-Reporting and Data System (CAD-RADS): Prognostic and Clinical Implications Associated With Standardized Coronary Computed Tomography Angiography Reporting. JACC Cardiovasc Imaging 2018; 11(1):78–89. [DOI] [PubMed] [Google Scholar]
- 4.Williams MC, Moss A, Dweck M, et al. Standardized reporting systems for computed tomography coronary angiography and calcium scoring: A real-world validation of CAD-RADS and CAC-DRS in patients with stable chest pain. J Cardiovasc Comput Tomogr 2020; 14(1):3–11. [DOI] [PubMed] [Google Scholar]
- 5.Basha MAA, Aly SA, Ismail AAA, Bahaaeldin HA, Shehata SM. The validity and applicability of CAD-RADS in the management of patients with coronary artery disease. Insights Imaging 2019; 10(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mushtaq S, Andreini D, Pontone G, et al. Prognostic value of coronary CTA in coronary bypass patients: a long-term follow-up study. JACC Cardiovasc Imaging 2014; 7(6):580–589. [DOI] [PubMed] [Google Scholar]
- 7.Chow BJW, Ahmed O, Small G, et al. Prognostic value of CT angiography in coronary bypass patients. JACC Cardiovasc Imaging 2011; 4(5):496–502. [DOI] [PubMed] [Google Scholar]
- 8.Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation 1956; 14(5):800–805. [DOI] [PubMed] [Google Scholar]
- 9.Graidis C, Dimitriadis D, Karasavvidis V, et al. Prevalence and characteristics of coronary artery anomalies in an adult population undergoing multidetector-row computed tomography for the evaluation of coronary artery disease. BMC Cardiovasc Disord 2015; 15(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 139(14):e698–e80.[Published correction appears in Circulation 2019;139(14):e833–e834.]. [DOI] [PubMed] [Google Scholar]
- 11.Hayes SN, Kim ESH, Saw J, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S, Manjunath L, Willemink MJ, Nieman K. The role of coronary CT angiography for acute chest pain in the era of high-sensitivity troponins. J Cardiovasc Comput Tomogr 2019; 13(5):267–273. [DOI] [PubMed] [Google Scholar]
- 13.Ghoshhajra BB, Takx RAP, Staziaki PV, et al. Clinical implementation of an emergency department coronary computed tomographic angiography protocol for triage of patients with suspected acute coronary syndrome. Eur Radiol 2017; 27(7):2784–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]



![Timeline plots of total quarterly PubMed citations resulting from the search “CAD-RADS”[Title/Abstract] OR “CADRADS”[Title/Abstract]. The date of the search was January 25, 2021.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/4767/8250406/d302b79a28e4/ryct.2021210016.fig1.jpg)