Dear Editor,
Both diagnostic laboratory samples and convalescent plasma (CP) are usually preserved frozen at temperatures below −25°C for long‐term storage. Nevertheless, investigating the stability of anti‐SARS‐CoV‐2 antibodies at refrigerator temperature (+4°C) is of paramount importance in logistical settings where freezers are not available, or when usage cannot be accomplished within time limits after thawing imposed by law (usually 1–5 days, to preserve stability of labile clotting factors, as currently recommended by the European Commission) and plasma refreezing is not allowed by law.
We repeated anti‐SARS‐CoV‐2 Spike (S) protein S1 subunit IgG and IgA testing with Euroimmun ELISA anti‐SARS‐CoV‐2 kits (Euroimmun Medizinische Labordiagnostika AG, Lubeck, Germany) on 24 residual diagnostic serum samples stored at +4°C for variable amount of time after the initial determination, without any freeze/thaw cycle. The study was approved by the internal review board (protocol 17437/2020). Statistical analyses were run using Spearman's Rho test on SPSS software v.23. The result plotted in Figure 1, lower panel, shows that both IgG and IgA levels (expressed as ratio between sample and calibrator) linearly declined by up to 30% at day 95. There was no correlation between intensity of reduction and baseline antibody levels (Figure 1, upper panel), and, as per manufacturer's instructions for user, the intra‐laboratory coefficient of variation for the assay run in the same lab at different timepoints is lower than 8% (data not shown). Preliminary reports by Stadlbauer et al. 1 showed stable IgG levels in 15 plasma samples for up to 42 days using an in‐house ELISA targeting the Spike protein: no details were disclosed regarding the kinetics of different immunoglobulin isotypes or the exact domain targeted by the assay. Our findings were instead achieved with a commercially available assay targeting the S1 subunit of the Spike protein: we extend the observation to 100 days, and for the first time report the kinetics of IgA isotype.
FIGURE 1.
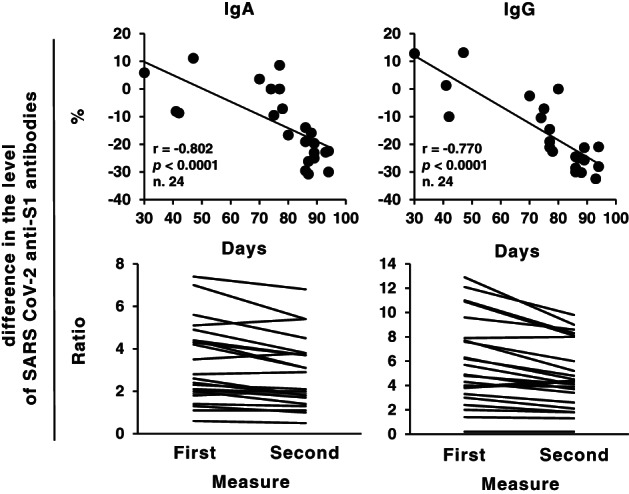
Kinetics of IgG and IgA antibody levels against SARS‐CoV‐2S1 subunit expressed either as % difference (lower panel) or absolute values of ratio (upper panel)
Previous studies on antibody stability in serum or plasma at 4°C are very scarce and old and mostly related to whole blood cells units, where anti‐cytomegalovirus IgG, IgA and IgM mean decrease for the fluorescence signals at week 8 was 1.2% both in serum and plasma. 2 Hodgkinsons et al. reported that IgG, IgA and IgM to epitopes from various viruses can be measured reliably from serum and plasma 4°C for up to 6 days before processing. 3 Similarly, anti‐CMV IgG were stable at day 14 in packed red blood cell units stored at 4°C. 4
The main limitations of our study are usage of serum rather than plasma samples (but no variations in antibody levels are seen between the two matrices in our experience) and reliance over paired testing of different sera rather than on sequential multiple testing of the same serum. Additionally, the implications for CP therapy, whose efficacy is largely based on neutralising antibody (nAb) levels, should be better assessed using a virus neutralisation test: nevertheless, receiver operating characteristic curve analysis showed Euroimmun ELISA area under the curve outperformed six different in‐house ELISAs and pseudotyped microneutralization test at predicting nAb titres >1:100 against the native isolate. A cut‐off value of 9.1 S/CO in the Euroimmun ELISA identified 65% of donations above the 1:100 nAb threshold, with no false identification of donations below this nAb threshold. 5
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTION
Daniele Focosi and Fabrizio Maggi designed the study and performed statistical analyses. Giovanna Moscato performed the serological assays. Mauro Pistello approved the final version.
REFERENCES
- 1. Stadlbauer D, Baine I, Amanat F, et al. Anti‐SARS‐CoV ‐2 spike antibodies are stable in convalescent plasma when stored at 4° Celsius for at least 6 weeks. Transfusion. 2020;60(10):2457‐2459. 10.1111/trf.16047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pappin A, Grissom M, Mackay W, Huang Y, Yomtovian R. Stability of cytomegalovirus antibodies in plasma during prolonged storage of blood components. Clin Diagn Lab Immunol. 1995;2:25‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hodgkinson VS, Egger S, Betsou F, et al. Preanalytical stability of antibodies to pathogenic antigens. Cancer Epidemiol BiomarkPrev. 2017;26:1337‐1344. [DOI] [PubMed] [Google Scholar]
- 4. Klinedinst AF, Baldwin ML, Ness PM. Detection of cytomegalovirus antibody in stored blood products using latex agglutination. Transfusion. 1988;28:563‐565. [DOI] [PubMed] [Google Scholar]
- 5. Harvala H, Robb ML, Watkins N, et al. Convalescent plasma therapy for the treatment of patients with COVID ‐19: Assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfus Med. 2020;31(3):167‐175. 10.1111/tme.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]


