Abstract
Chimeric antigen receptor (CAR) T cells directed against CD19 (CD19.CAR T cells) have yielded impressive clinical responses in the treatment of patients with lymphoid malignancies. However, resistance and/or relapse can limit treatment outcome. Risk of tumor escape can be reduced by combining treatment strategies. Selective inhibitors of nuclear export (SINEs) directed against nuclear exportin-1 (XPO1) have demonstrated anti-tumor efficacy in several hematological malignancies. The aim of the present study was to evaluate the combination of CAR T cells with the SINE compounds eltanexor and selinexor. As expected, eltanexor and selinexor were toxic to CD19-positive malignant cells and the sensitivity of cells towards SINEs correlated with the levels of XPO1-expression in ALL cell lines. When SINEs and CAR T cells were simultaneously combined, SINEs exerted toxicity towards CAR T cells and impaired their function affecting cytotoxicity and cytokine release ability. Flow cytometry and western blot analysis revealed that eltanexor decreased the cytoplasmic concentration of the transcription factor phosphorylated-STAT3 in CAR T cells. Due to CAR T-cell toxicity, sequential use of SINEs and CAR T cells was evaluated: Cytotoxicity of CAR T cells increased significantly when target cells were pre-treated with the SINE compound eltanexor. In addition, exhaustion of CAR T cells decreased when target cells were pre-treated with eltanexor. In summary, whereas the concomitant use of SINEs and CAR T cells does not seem advisable, sequential use of SINEs and CAR T cells might improve the anti-tumor efficacy of CAR T cells.
Keywords: CD19, chimeric antigen receptor, T cells, selective nuclear export inhibitor, selinexor, eltanexor, STAT3, nuclear exportin-1
Introduction
Chimeric antigen receptor (CAR) T cells are individualized living drugs which combine the properties of T lymphocytes with the specificity of antibodies. They represent potent weapons to treat malignancies (1,2). CAR T cells directed against CD19 (CD19.CAR T cells) have shown remarkable clinical results in heavily pre-treated patients with relapsed or refractory (r/r) lymphoid malignancies (3–6), including acute lymphoblastic leukemia (ALL) (7,8), chronic lymphocytic leukemia (CLL) (9,10) and non-Hodgkin's lymphoma (NHL) (11,12). In fact, several CAR T-cell products have been approved by the regulatory authorities and have been adopted as standard of care within the labelled indications (13–16). However, antigen-positive as well as antigen-negative relapses and resistance to treatment are commonly observed in ALL (17–19), CLL (10) and NHL (20) patients following CD19.CAR T-cell treatment. Considering that cancer therapy against only a single target may facilitate the development of resistance, combining antigen-specific CAR T cells with less specific anti-tumor agents may overcome resistance to treatment, prevent disease relapse and enhance anti-tumor responses in patients.
Exportin-1 (XPO1), also termed chromosome region maintenance 1 (CRM1), is a nuclear export receptor involved in the transportation of proteins such as histones, polymerases, transcription factors and/or RNA from the nucleus into the cytosol (21). Notably, the export of tumor suppressor proteins (TSPs) depends on XPO1 (22,23). Hematological as well as solid malignancies overexpress XPO1 (24–31) to limit nuclear TSP effects and evade inherent tumor control. XPO1 overexpression has been observed in aggressive diseases, and elevated XPO1 levels have been associated with poor clinical outcome in numerous neoplasms (32–36). Thus, downregulation of XPO1 constitutes an interesting therapeutic strategy. Notably, inhibiting XPO1 by selective inhibitors of nuclear export (SINEs) has been shown to restore and enhance the function of TSPs (21), and anti-tumor efficacy of SINEs has been demonstrated in hematological malignancies including multiple myeloma (MM) (32), ALL (33), NHL (34,35), acute myeloid leukemia (AML) (36) as well as in solid tumors (24–26). The SINE compound selinexor was approved for the treatment of adults with r/r MM by the U.S. Food and Drug Administration (FDA) in September 2019, and by the European Medicines Agency (EMA) in December 2019 (37). Moreover, selinexor is currently under clinical evaluation for treatment of diffuse large B-cell lymphoma (DLBCL; NCT02227251), r/r AML and myelodysplastic syndrome (MDS; NCT03071276) as well as advanced liposarcoma (NCT02606461).
Given that protein transport regulation through XPO1 across the nuclear membrane is essential to normal cells (36), SINEs disturb normal immune homeostasis resulting in side effects such as cytopenia. Selinexor can cross the blood-brain-barrier (BBB) and cause central anorexia with associated weight loss and malaise (36,38). The second-generation SINE eltanexor has a reduced effect on hematopoietic stem and progenitor (HSPCs) cells (39) and an approximately 30-fold lower capacity to penetrate the BBB than selinexor (32). Therefore, eltanexor has a more favorable side effect profile when compared to selinexor, while maintaining anti-tumor efficacy. Eltanexor has shown potent anti-lymphoblastic activity in pre-clinical patient-derived T- and B-ALL xenograft models (32).
The aim of the present study was to investigate the impact of the SINE compounds selinexor and eltanexor on tumor cells as well as third-generation CAR T cells and to evaluate potential combinatorial effects of eltanexor and CAR T cells.
Materials and methods
Peripheral bood mononuclear cell (PBMCs)
Peripheral blood mononuclear cells (PBMCs) of seven healthy donors (HDs) were collected at the Heidelberg University Hospital, Heidelberg, Germany. Sample collection and analysis were approved by the Ethics Committee of the University of Heidelberg (S-254/2016) and all donors signed a written consent prior to treatment. All experiments were performed in accordance with the convention of Helsinki.
Cell lines
Burkitt lymphoma cell lines Daudi and Raji as well as ALL cell lines Nalm-6 and Reh [German Collection of Microorganisms and Cell Cultures (DSMZ)] were used as CD19-positive CAR T-cell target cells. Chronic myelogenous leukemia (CML) cell line K562 (DSMZ) was used as CD19-negative control cell line. 293T cells were obtained from the American Type Culture Collection (ATCC). Cells were cultured in RPMI-1640 (Thermo Fisher Scientific) supplemented with 2 mM L-glutamine (Thermo Fisher Scientific) and 10% heat-inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific) at 37°C and 5% CO2.
Cell culturing
Due to the experimental design, co-culturing experiments of CAR T cells (effector cells) with tumor cell lines (Nalm-6, Daudi, Raji and Reh) and eltanexor were performed using two different culture conditions: i) simultaneous co-culturing of CAR T cells, target cells and eltanexor, or ii) pre-treatment of target cells with eltanexor (0.05, 0.1 and 0.5 µM) and washing-out of eltanexor prior to addition of CAR T cells.
CAR T-cell generation
Retrovirus generation and CD19 CAR transfection
The third-generation retroviral vector RV-SFG.CD19.CD28.4-1BB.CD3zeta used in a CD19.CAR T-cell clinical trial conducted at the University Hospital Heidelberg (EudraCT 2016-004808-60; NCT03676504) comprising CD28 and 4-1BB (CD137) as costimulatory domains (40) was used for CAR T-cell manufacturing. Retroviral supernatant was generated via transfection of 293T cells with three plasmids: i) plasmid RV-SFG.CD19.CD28.4-1BB.CD3zeta (3.75 µg) containing the CD19-specific CAR transgene, ii) packaging plasmid PegPam3 containing gag-pol (3.75 µg) and iii) the envelope plasmid RDF plasmid containing env (2.5 µg). SFG.CD19.CD28.4-1BB.CD3zeta, PegPam3 and RDF plasmids were kindly provided by Professor Malcolm Brenner, Center for Cell and Gene Therapy, Houston, TX, USA. Details of retrovirus generation and transfection have been previously described (41,42).
CAR T-cell manufacturing
CAR T cells were manufactured as previously described (41,42). In brief, on day 0, cryopreserved PBMCs from HDs were thawed and seeded on anti-CD3- and anti-CD28 coated 24-well plates (Corning). On day 3, activated T cells (ATCs) supplied with retroviral supernatant were transferred into 24-well plates (Corning) previously coated with retronectin (Takara Bio). Efficacy was evaluated on days 7, 10, 14 and 17 after transduction using flow cytometry.
SINE compounds
Selinexor (KPT330) and eltanexor (KPT8602) (Selleck Chemicals) were dissolved in DMSO to a stock concentration of 10 mmol/l.
CellTiter-Glo assay
Viability assay CellTiter-Glo (Promega, Fitchburg) was used for cell number titration as well as subsequent compound titration. After adding CellTiter-Glo buffer to the CellTiter-Glo substrate (Promega) to reconstitute the lyophilized enzyme/substrate mixture, CellTiter-Glo reagent was aliquoted and stored at −20°C until use. CellTiter-Glo experiments including cell number titration and SINE compound titration were performed sequentially.
Cell number titration
To obtain cells growing in the logarithmic phase at 48 h, tumor cell lines (Daudi, Raji, Nalm-6, Reh), CAR T cells, and non-transduced T cells (negative control) were added to 384-well plates (Greiner Bio-One) and diluted 1:1.5 with RPMI-1640 medium (tumor cell lines) or with complete medium (T cells).
Compound titration on tumor cell lines and CAR T cells
To assess the effects of SINEs on tumor cells as well as CAR T cells, effects of selinexor and eltanexor on cell viability were analyzed.
The therapeutic window of SINEs was assessed via compound titration: Following cell number titration, selinexor and eltanexor or DMSO as control were added at concentrations from 10 to 0.001 µM (dilution of eltanexor and selinexor performed with phosphate-buffered saline (PBS) at ratios of 1:3) to Daudi, Raji, Nalm-6, Reh, CAR T cells as well as non-transduced T cells in 384-well plates (Greiner Bio-One) and the half-maximal inhibitory concentrations (IC50) determined.
Tumor or CAR T cells with added SINEs were cultivated for 48 h in 384-well plates (Greiner Bio-One). After cultivation, 12 µl of CellTiter-Glo reagents, i.e., CellTiter-Glo and substrate (Promega), were added into each well of the culturing system. The mixture of the solution was incubated for 15–20 min at room temperature (RT) and luminescence was recorded by the Ensight Multimode Plate Reader (PerkinElmer). Relative viability of serial dilutions was used to calculate the IC50.
Flow cytometric analysis
According to the location of expression of analyzed markers, surface marker staining or intracellular staining (ICS) was performed using the FoxP3 staining buffer set (cat. no. 130-093-142, Miltenyi Biotec) at 4°C for 6 h. For all staining procedures, dead cells were excluded using the LIVE/DEAD fixable near-infrared (IR) dead cell stain kit (Thermo Fisher Scientific). Anti-human goat F(ab) IgG (H+L) PE antibody (cat. 109-116-088; Dianova) was used to distinguish CD19-specific CAR T cells from non-transduced T cells. After staining, all the samples were measured on the flow cytometer LSRII (BD Biosciences) and data were analyzed using FlowJo.
Surface marker staining
The following antibodies were used for surface marker staining: Anti-human goat F(ab) IgG (H+L) PE antibody (cat. no. 109-116-088) from Dianova; anti-CD3-PE eFluor 610 (cat. no. 61-0038-42), and anti-CD4-Alexa Fluor 700 (cat. no. 560049-42) from eBioscience, San Diego; anti-CD8-PerCP (cat. no. 344708), anti-CD10-APC (cat. no. 312210), anti-human CD223-APC (LAG-3, Cat. 369212), anti-PD-1-Alexa Fluor 488 (cat. no. 329935), anti-human CD366 (Tim-3, Cat. 345007) all from Biolegend; anti-CD3-V500 (cat. no. 561416) from BD Biosciences.
ICS for evaluation of cytokine release by CAR T cells
For cytokine release assessment, CAR T cells with or without the addition of different doses of eltanexor were stimulated by incubation with CD19-positive target cells for 6 h in 96-well U-bottom microplates (Greiner BioOne) in the presence of Brefeldin A (Biolegend). The cell mixture was fixated and permeabilized using the FoxP3 staining buffer set (cat. no. 130-093-142, Miltenyi Biotec). Cells were incubated for 30 min at RT in the dark for fixation with fixation/permeabilization solution (fixation/permeabilization solution 1: Fixation/permeabilization solution 2=1:4) and 15 min at RT for permeabilization with the FoxP3 permeabilization buffer. ICS was performed with anti-interferon (IFN)-γ-Alexa Fluor 488 (cat. no. 502515; Biolegend) and anti-tumor necrosis factor (TNF)-α-BV421 (cat. no. 562783; BD Biosciences).
ICS for evaluation of phosphorylated-STAT3
For staining of phosphorylated-STAT3, CAR T cells were fixed by incubation with fixation buffer (Biolegend) for 15 min at RT, before they were permeabilized with True-Phos perm buffer (Biolegend) at −20°C overnight. The anti-STAT3 phosphorylated (Tyr705) antibody (Biolegend) was added to stain phosphorylated-STAT3.
Flow cytometric analysis used to evaluate cytotoxicity of CAR T cells towards Nalm-6 cells
As Nalm-6 cells have a low Chromium 51 (51Cr) ∆release (∆release=maximum release-spontaneous release), Cr release (mentioned in a section below) is an inadequate method to assess the cytotoxicity of CAR T cells towards Nalm-6 cells (Fig. S1). Consequently, flow cytometric analysis was used to evaluate cytotoxicity of CAR T cells towards Nalm-6 cells. After either simultaneous co-culturing (CAR T cells, Nalm-6 cells and eltanexor) or culturing of CAR T cells with pre-treated Nalm-6 cells, cells were collected and stained with the following antibodies: Anti-human goat F(ab) IgG (H+L) PE antibody (Dianova), anti-CD3-PE eFluor 610 (eBioscience), anti-human CD223 (LAG-3), anti-PD-1-Alexa Fluor 488, anti-human CD366 (Tim-3) (Biolegend) and flow cytometry was performed.
Chromium 51 release assay
51Cr release assay to address functionality of CAR T cells towards Daudi, Raji or Reh cells was performed as previously described (43,44). Effector to target cell ratios of 10:1, 5:1, 2.5:1 and 1:1 were used.
Pre-treating tumor cell lines with eltanexor and CAR T cells
Tumor cells were labeled with 51Cr (Hartmann Analytic) for 2 h in a humidified incubator at 37°C and 5% CO2. Subsequently, CAR T cells were added and co-culturing was performed for 4 h at 37°C and 5% CO2 in a 96-well U-bottom microplate (Greiner Bio-One). The supernatant was collected to perform radioactive activity measurement as previously described (41,42).
Simultaneous co-culturing of tumor cell lines, CAR T cells and eltanexor
Either Daudi, Raji or Reh cells were labeled with 51Cr for 2 h in a humidified incubator at 37°C and 5% CO2. After labeling, the cells were co-cultured with CAR T cells, followed by the addition of eltanexor at different concentrations (0.05, 0.1 and 0.5 µM). The negative control contained DMSO instead of eltanexor. The cells and eltanexor were cultured in 96-well U-bottom microplates (Greiner Bio-One) for 4 h at 37°C and 5% CO2. The supernatant was collected to perform radioactive activity measurement as previously described (41,42).
Western blot analysis
One million CD19 CAR T cells, non-transduced T cells and tumor cells, respectively, with or without the addition of eltanexor were lysed in 200 µl radio-immunoprecipitation assay buffer (RIPA buffer; Thermo Fisher Scientific) after the addition of complete protease inhibitor (Sigma-Aldrich; Merck KGaA) at RT for 10 min followed by centrifugation for 10 min at 12.000 × g at 4°C. Protein-containing supernatants were collected and 20 µg protein was loaded on a 4–12% SDS-PAGE gel. Separated proteins were immediately blotted onto nitrocellulose membranes. Prior to incubation with a primary antibody at a dilution of 1:200 (anti-exportin-1/CRM1, anti-phosphorylated-STAT3 (only for CAR T cells) or 1:500 [anti-beta (β) actin (as internal reference), anti-total STAT3 (only for CAR T cells)] at 4°C overnight, the membranes were blocked for 1 h at RT with 5% milk in Tris-buffered saline with Tween-20 (TBST). Appropriate horseradish peroxidase-conjugated secondary antibodies (anti-mouse IgG or anti-rabbit IgG, HRP-linked antibody (Cell Signaling Technology, Frankfurt) were used at a dilution of 1:2,000. Proteins were visualized in an Amersham Imager 600 (GE Healthcare). Quantification of the of protein bands was performed using software ImageJ.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 6 (GraphPad Software Inc.). P-values were calculated using the parametric two-way t-test between two groups, and the one-way analysis of variance (ANOVA) with Bonferroni's multiple comparison test for three or four groups. P<0.05 was considered statistically significant. When not otherwise indicated, results were represented as mean ± standard deviation (SD). IC50s were presented as mean ± standard error of the mean (SEM). Graphs and tables were designed using GraphPad Prism 6.
Results
Sensitivity of tumor cells towards selinexor and eltanexor and measuring of XPO1 protein levels
In order to improve CAR T cell efficacy and overcome refractory disease, we evaluated the combination of CAR T cells with the SINE compounds selinexor and eltanexor.
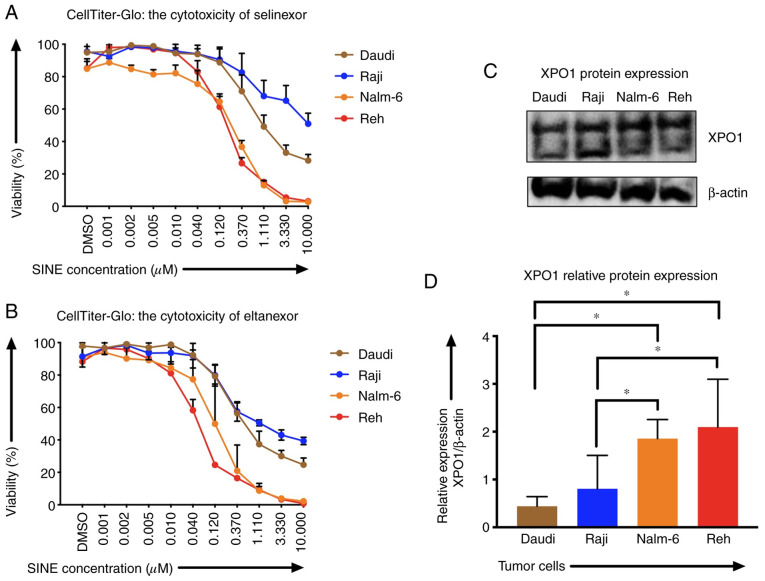
Sensitivities of Reh, Nalm-6, Daudi and Raji cells to SINEs were analyzed assessing IC50 of selinexor and eltanexor. Selinexor and eltanexor effectively inhibited viabilities of Reh [IC50: Selinexor: 0.16±0.01 µM (Fig. 1A), eltanexor: 0.05±0.01 µM (Fig. 1B)] and Nalm-6 cells [IC50: Selinexor: 0.30±0.02 µM (Fig. 1A), eltanexor: 0.14±0.03 µM (Fig. 1B)]. Daudi cells showed medium sensitivity [selinexor: 0.60±0.09 µM (Fig. 1A), eltanexor: 0.30±0.03 µM (Fig. 1B)], whereas Raji cells exhibited the lowest sensitivity to SINEs [IC50s: Selinexor: 1.33±1.16 µM (Fig. 1A), eltanexor: 0.23±0.03 µM (Fig. 1B)].
Figure 1.
XPO1 expression and sensitivity of CD19-positive tumor cells Nalm-6, Reh, Daudi and Raji towards SINEs selinexor and eltanexor. To analyze the efficacy of varying concentrations of (A) selinexor and (B) eltanexor on cells, half-maximal inhibitory concentrations (IC50) were assessed using the viability assay CellTiter-Glo. The initial concentration of both selinexor and eltanexor was 10 µM; both agents were diluted with phosphate-buffered saline (PBS) sequentially at ratios of 1:3, until the minimum concentration of 0.001 µM was reached. DMSO was used as negative control. Nalm-6 (orange line) and Reh (red line) cells displayed the highest sensitivity to selinexor and eltanexor when compared to Daudi (brown line) cells. Raji (blue line) cells showed the lowest sensitivity to SINEs. XPO1 protein expression levels (C) of Nalm-6, Reh, Daudi and Raji cells were assessed by western blot analysis, β-actin was used as internal reference. Relative XPO1 expression was calculated. (D) Nalm-6 and Reh cells showed the highest XPO1 expression when compared to Daudi and Raji cells. Experiments were performed in duplicate. IC50 is presented as the mean ± standard error of the mean (SEM). *P<0.05 indicates statistical significance.
Sensitivity of ALL and NHL tumor cells towards selinexor and eltanexor was associated with the protein levels of the SINE target CRM1/XPO1: ALL cells Reh (2.10±0.01) and Nalm-6 (1.86±0.01), that had shown the highest sensitivity to SINEs, displayed higher relative XPO1 protein levels compared to the NHL cells Daudi (0.44±0.01) and Raji (0.81±0.01) (Fig. 1C and D) (P<0.05).
Tumor cells were more sensitive to eltanexor, suggesting a superior toxicity profile of eltanexor compared to selinexor (32,39). Consequently, eltanexor was chosen as SINE compound to perform further experiments.
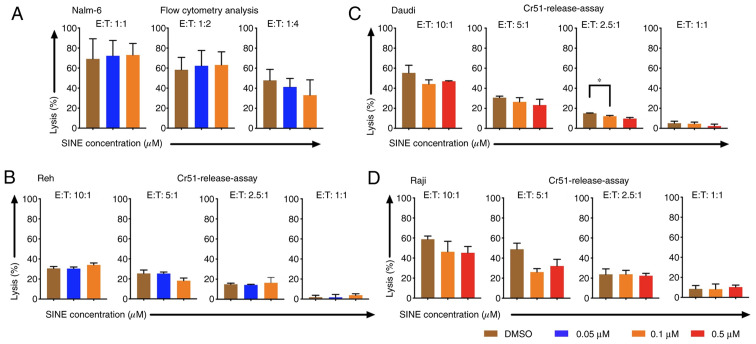
Cytotoxicity of CAR T cells was abrogated when CAR T cells and tumor cells were cultivated concomitantly with eltanexor
Cytotoxicity of CAR T cells towards tumor cells was addressed when they were cultivated simultaneously with target cells and eltanexor. Nalm-6, Reh, Daudi and Raji cells were co-cultured with eltanexor at their respective IC50 (0.05 and 0.1 µM for Nalm-6 and Reh; 0.1 and 0.5 µM for Daudi and Raji) as well as CAR T cells. Simultaneous co-culturing decreased cytotoxicity of CAR T cells towards Nalm-6 (Fig. 2A, assessed via flow cytometry), Reh (Fig. 2B, assessed via 51Cr release assay) (0.05 µM eltanexor used), Daudi (Fig. 2C, assessed via 51Cr release assay) as well as Raji (Fig. 2D, assessed via 51Cr release assay) (0.1 µM eltanexor used) cells compared to DMSO [Nalm-6: 1:1 ratio: 72.3 vs. 69.2%; 1:2 ratio: 62.3 vs. 58.3%; 1:4 ratio: 41.3 vs. 47.8% (Fig. 2A); Reh: 10:1 ratio: 30.4±1.6 vs. 30.7±1.9%, 5:1 ratio: 25.4±1.5 vs. 25.5±3.4%, 1:1 ratio: 1.7±3.0 vs. 1.8±1.8% (Fig. 2B). Daudi cells: 10:1 ratio: 44.2±4.2 vs. 55.2±7.6%, 5:1 ratio: 26.5±4.2 vs. 30.7±1.4%, 2.5:1 ratio: 12.2±0.7 vs. 15.0±0.3% (P=0.0088), 1:1 ratio: 4.6±1.6 vs. 5.1±2.0% (Fig. 2C) and Raji cells: 10:1 ratio: 46.3±10.5 vs. 58.8±3.2%, 5:1 ratio: 26.1±3.4 vs. 48.8±6.1%, 2.5:1 ratio: 23.8±5.4 vs. 23.8±3.9%, 1:1 ratio: 8.2±5.p =2% vs. 8.5±3.4%) (Fig. 2D)].
Figure 2.
Cytotoxicity of CAR T cells towards tumor cells Nalm-6, Reh, Daudi and Raji when CAR T cells were cultivated simultaneously with the SINE compound eltanexor (0.05 µM: Blue, 0.1 µM: Orange, 0.5 µM: Red) and tumor cells. Nalm-6 cells (A) were co-cultured for 24 h with eltanexor and CAR T cells before cytotoxic potential of CAR T cells was assessed via flow cytometry analysis. Reh (B) Daudi (C) and Raji (D) cells were co-cultured for 4 h with eltanexor and CAR T cells before cytotoxic capacity of CAR T cells was evaluated via chromium release. DMSO was used as a negative control. A trend towards decreased lytic efficacy of CAR T cells was assessed on Reh (B) Daudi (C) and Raji (D) cells when cultured simultaneously with eltanexor compared to the DMSO control (statistically not significant). Experiments were performed in triplicate. Results are presented as mean ± standard deviation (*P<0.05 indicates statistical significance).
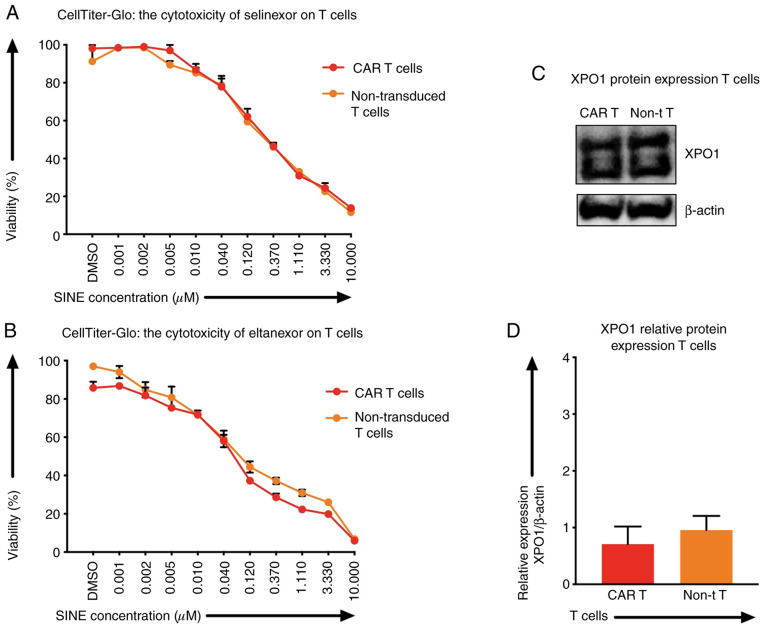
XPO1 protein levels and the sensitivity of T cells towards SINEs
Besides tumor cells, selinexor and eltanexor also inhibited viabilities of T cells, i.e., CAR T cells [IC50: Selinexor (0.20±0.04 µM) (Fig. 3A, red line), eltanexor (0.06±0.02 µM) (Fig. 3B, red line)] and non-transduced T cells [IC50s: Selinexor (0.28±0.08 µM) (Fig. 3A, orange line), eltanexor (0.04±0.05 µM) (Fig. 3B, orange line)]. The protein levels of XPO1 in CAR T cells and non-transduced T cells were quantified by western blot analysis: The relative expression of XPO1 protein of CAR T cells was 0.71±0.01 and of non-transduced T cells 0.96±0.01 (Fig. 3C and D). Differences of XPO1 protein levels and differences of sensitivity of CAR T cells and non-transduced T cells towards selinexor or eltanexor were not statistically significant (Fig. 3C).
Figure 3.
XPO1 protein levels and sensitivity of T cells (CAR T cells and non-transduced T cells) towards SINEs. To assess the efficacy of varying concentrations of selinexor (A) and eltanexor (B) on T cells, half-maximal inhibitory concentrations (IC50) were assessed using the viability assay CellTiter-Glo. The initial concentration of both selinexor and eltanexor was 10 µM. The two agents were diluted with phosphate-buffered saline (PBS) sequentially at ratios of 1:3, until the minimum concentration of 0.001 µM was reached. DMSO was used as negative control. No toxicity difference of CAR T cells and non-transduced T cells towards selinexor or eltanexor was observed. XPO1 protein levels (C) and relative XPO1 expression (D) of T cells [CAR T cells (red), non-transduced T cells (orange)] were assessed by western blot analysis; β-actin was used as the internal reference. No significant difference of XPO1 protein levels of CAR T cells was observed when compared to non-transduced T cells. Experiments were performed in duplicate. IC50 is represented as the mean ± standar error of the mean (SEM).
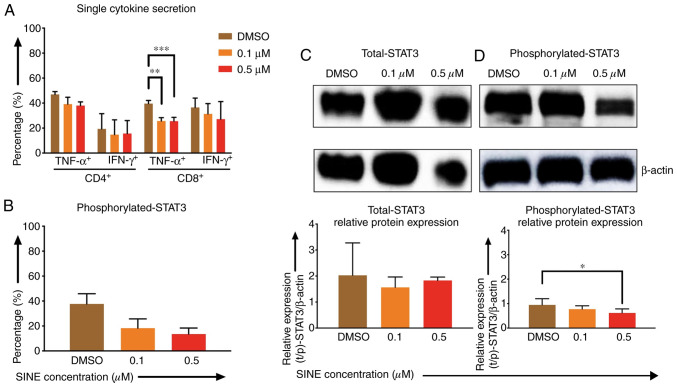
Effects of eltanexor on CAR T cells were pronounced when CAR T cells were cultivated simultaneously with Daudi cells and eltanexor
Lower cytokine secretion of TNF-α and IFN-γ by CD4- and CD8-positive CAR T cells after stimulation with Daudi cells and eltanexor (0.1 and 0.5 µM) was observed when compared to the DMSO control: CD4 TNF-α+: 39.2±5.5%, 38.0±3.0% vs. 46.9±2.3%; CD4+ IFN-γ+: 14.8±11.8%, 15.6±10.4% vs. 19.4±12.2%; CD8+ TNF-α+: 25.7±2.7% (P=0.0007), 25.5±3.1% (P=0.0012) vs. 39.6±2.6%; CD8 IFN-γ+: 31.3±8.3%, 21.1±14.1% vs. 36.6±7.5% (Fig. 4A).
Figure 4.
Effects of SINEs when eltanexor, CAR T cells and Daudi cells were cultivated simultaneously. Daudi cells were cultivated simultaneously with CAR T cells and eltanexor (0.1 µM: Orange, 0.5 µM: Red) or with DMSO as control (brown) for 6 h (A, assessed via flow cytometry analysis) or 4 h (B, assessed via flow cytometry analysis, C and D via western blot analysis). Cytokine release (IFN-γ and TNF-α) of CD4- and CD8-positive CAR T cells was assessed and decreased cytokine secretion after stimulation of CAR T cells with Daudi cells compared to the DMSO control was observed. (A) Phosphorylated-STAT3 level of CAR T cells was evaluated. Decreased phosphorylated-STAT3 of CAR T cells cultured with eltanexor was observed when compared to the DMSO control. (B) Evaluation of total STAT3 and phosphorylated-STAT3 protein levels of CAR T cells was performed after protein isolation via western blot analysis on STAT3 of CAR T cells; β-actin was used as the internal reference. Raw total STAT3 protein level (C, upper panel), relative STAT3 expression (C, lower panel), raw phosphorylated-STAT3 protein level (D, upper panel) and relative phosphorylated-STAT3 expression (D, lower panel) were assessed. The protein levels of phosphorylated-STAT3 compared to total STAT3 were decreased. Experiments were performed in triplicate. Mean values were calculated for each group; results are presented as mean ± standard deviation (SD). *P<0.05, **P<0.005, ***P<0.0005 indicates statistical significance.
Phosphorylated-STAT3 (p-STAT3) levels of CAR T cells when co-cultured with eltanexor were assessed via flow cytometry: p-STAT3 decreased significantly when eltanexor was used within the culture [0.1 and 0.5 µM eltanexor vs. DMSO control: 18.3±7.4% (P=0.0244), 13.5±4.8% (P=0.0094) vs. 37.1±8.1% (Fig. 4B)]. To further verify the decrease of p-STAT3 in the cytoplasm of CAR T cells, difference in protein levels of total STAT3 and phosphorylated STAT3 was quantified by western blot analysis. Total STAT3 protein levels showed no difference (Fig. 4C). However, compared to the DMSO control, 0.1 and 0.5 µM of eltanexor demonstrated a decreased phosphorylated STAT3 protein expression in the cytoplasm: 0.1 and 0.5 µM vs. DMSO: 0.8±0.1% (P=0.2932), 0.4±0.2% (P=0.0281), vs. 0.9±0.3% (Fig. 4D).
Cytotoxicity of CAR T cells was improved when tumor cells were pre-treated with eltanexor
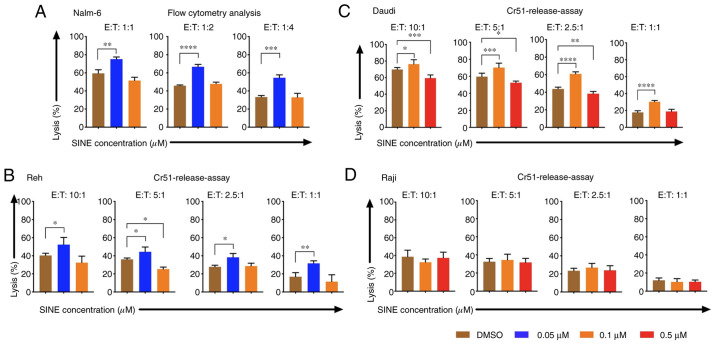
Pre-sensitizing of tumor cells with eltanexor before CAR T-cell exposure was addressed: Nalm-6, Reh, Daudi as well as Raji cells were pre-treated with eltanexor (0.05 and 0.1 µM for Nalm-6 and Reh; 0.1 and 0.5 µM for Daudi and Raji) and eltanexor was removed by additional washing with culturing medium before CAR T cells were added. Pre-treated Nalm-6 cells were cultivated with CAR T cells for 24 h. Assessment of cytotoxicity of CAR T cells was performed via flow cytometry as 51Cr release assay was not adequate to evaluate the toxicity of CAR T cells towards Nalm-6 cells (Fig. S1). Toxicity of CAR T cells on Reh, Daudi and Raji cells was assessed via 51Cr release assay. Pre-treatment with 0.05 µM eltanexor significantly increased cytotoxicity of CAR T cells towards Nalm-6 and Reh cells as compared to DMSO [Fig. 5A and B: Nalm-6: 1:1 ratio: 75.1±2.5 vs. 59.3±4.1% (P=0.0025); 1:2 ratio: 66.7±2.7 vs. 45.8±0.9% (P<0.0001), 1:4 ratio: 54.5±3.3 vs. 33.3±1.7% (P=0.0004); Reh: 10:1 ratio: 52.4±8.0 vs. 40.3±2.4% (P=0.0163), 5:1 ratio: 44.3±5.4 vs. 36.1±1.5% (P=0.0472), 2.5:1 ratio: 38.3±4.3 vs. 27.8±1.8% (P=0.0130), 1:1 ratio: 31.6±2.9 vs. 16.9±4.5% (P=0.0025)]. The increase of cytotoxicity of CAR T cells was also observed when Daudi cells pretreated with 0.1 µM eltanexor were used as target cells [10:1 ratio: 76.3±5.3 vs. 69.9±2.3% (P=0.0278), 5:1 ratio: 70.3±5.2% vs. 59.7±4.0% (P=0.0007), 2.5:1 ratio: 61.0±2.3% vs. 43.8±2.1% (P<0.0001), 1:1 ratio: 31.6±1.6% vs. 17.7±2.0% (P<0.0001) (Fig. 5C)]. However, improvement of toxicity was not observed for CAR T cells towards pre-treated Raji cells with 0.1 or 0.5 µM eltanexor (Fig. 5D) that had previously shown the lowest sensitivity to SINEs (Fig. 1A). However, pre-treating tumor cells with high concentrations of eltanexor reversed the cytotoxicity of CAR T cells [Reh, 0.1 µM eltanexor vs. DMSO: 5:1 ratio: 25.3±2.3 vs. 36.1±1.5% (P=0.0159); Daudi, 0.5 µM eltanexor vs. DMSO: 10:1 ratio: 59.3±4.0 vs. 69.9±2.3% (P=0.0008), 5:1 ratio: 52.4±2.0 vs. 59.7±4.0% (P=0.0121), 2.5:1 ratio: 38.4±2.6 vs. 43.8±2.1% (P=0.0023) (Fig. 5B and C)]. This effect of reversing cytotoxicity of CAR T cells was also observed for Nalm-6 cells pre-treated with high concentrations of eltanexor, although this was not statistically significant [0.1 µM eltanexor vs. DMSO: 1:1 ratio: 51.5±3.6 vs. 59.3±4.1% (P=0.0533) (Fig. 5A)].
Figure 5.
Cytotoxicity of CAR T cells when target cells were pre-treated with the SINE compound eltanexor. Nalm-6 (A) Reh (B) Daudi (C) and Raji (D) cells were cultivated with eltanexor at different concentrations (0.05 µM: Blue, 0.1 µM: Orange, 0.5 µM: Red) or with DMSO as control (brown) for 24 h. After washing with medium, CAR T cells were added. Cytotoxicity of CAR T cells on Nalm-6 cells was assessed via flow cytometry following co-cultivation for 24 h. Reh, Daudi and Raji cells were co-cultured for 4 h with CAR T cells prior to evaluation of cytotoxicity of CAR T cells via chromium release. Increased cytotoxicity of CAR T cells was observed for Nalm-6, Reh and Daudi cells when low concentrations of eltanexor pre-treatment were used (0.05 µM on both Nalm-6 and Reh, 0.1 µM on Daudi) as compared to the DMSO control (brown). Higher concentrations of eltanexor (0.1 µM on Reh, 0.5 µM on Daudi) abrogated the lytic effects of CAR T cells. Improvement of lytic capacity after pretreatment with eltanexor was not observed for Raji cells. Experiments were performed in triplicate. Results are presented as mean ± standard deviation (SD). *P<0.05, **P<0.005, ***P<0.0005, ****P<0.00005 indicate statistical significance. E: Effector cells, i.e., CAR T cells; T: Target cells. i.e., Nalm-6, Reh, Daudi and Raji cells.
Cytokine release levels of CAR T cells increased when tumor cells were pre-treated with eltanexor
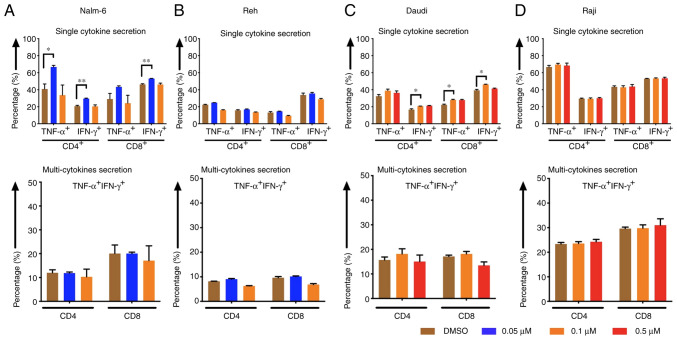
CD4- and CD8-positive CAR T cells displayed higher cytokine (TNF-α and IFN-γ) secretion levels after stimulation of CAR T cells with Nalm-6 and Daudi cells when target cells had been pre-treated with eltanexor [(Nalm-6: 0.05 µM eltanexor vs. DMSO): CD4 TNF-α+: 66.7±2.0 vs. 40.9±5.9% (P=0.0157), CD4 IFN-γ+: 29.6±0.6 vs. 20.8±0.9% (P=0.0093), CD8 TNF-α+: 43.3±1.3 vs. 29.0±6.6% (P=0.1272), CD8 IFN-γ+: 53.1±0.2 vs. 46.3±0.8% (P=0.0056) (Fig. 6A, upper panel); Daudi (0.1 µM eltanexor vs. DMSO): CD4 TNF-α+: 38.8±1.9 vs. 32.1±1.0% (P=0.1805), CD4 IFN-γ+: 20.7±0.2 vs. 16.6±1.0% (P=0.0299), CD8 TNF-α+: 28.0±0.7 vs. 22.3±0.8% (P=0.0467), CD8 IFN-γ+: 46.2±0.4 vs. 39.4±1.1% (P=0.0167) (Fig. 6C, upper panel)]. Although a trend towards an increase in multi-cytokine release (IFN-γ and TNF-α double positive) was also observed after stimulation of CAR T cells with pre-treated Nalm-6 (0.05 µM eltanexor) and Daudi (0.1 µM eltanexor) cells, this was not statistically significant (Fig. 6A and C, lower panel). An increase in secretion of single cytokine or multiple cytokines was also observed for Reh cells pre-treated with 0.1 µM eltanexor, but this was without statistical significance when compared to DMSO (Fig. 6B). Improved cytokine-secretion was not observed for CAR T cells stimulated with Raji cells (Fig. 6D).
Figure 6.
Cytokine release of CAR T cells after pre-treatment of tumor cells with the SINE compound eltanexor. Nalm-6 (A) Reh (B) Daudi (C) and Raji (D) cells were cultivated with eltanexor at different concentrations (0.05 µM: Blue, 0.1 µM: Orange, 0.5 µM: Red) or with DMSO as control (brown). After washing with culturing medium, CAR T cells were incubated with tumor cells for 6 h. Single cytokine release (IFN-γ and TNF-α) of CD4- and CD8-positive CAR T cells was assessed. Higher cytokine secretion levels were detected after stimulation of CAR T cells with Nalm-6 (pre-treated with 0.05 µM eltanexor, blue, A, upper panel) and Daudi cells (pre-treated with 0.1 µM eltanexor, orange, C, upper panel) when compared to the DMSO control (brown, A and C, upper panel). The increase was also observable when multi-cytokine secretion (IFN-γ and TNF-α double positive, A and C, lower panel) of CD4- and CD8-positive CAR T cells was measured, although this was not statistically significant. No alternation of cytokine secretion of CAR T cells towards pre-treated Raji cells (pre-treated with 0.1 and 0.5 µM eltanexor, D) was detected. Experiments were performed in triplicate. Results are presented as mean ± standard deviation (SD). *P<0.05 and **P<0.005 indicate statistical significance.
The expression of exhaustion markers of CAR T cells was decreased when tumor cells were pre-treated with eltanexor
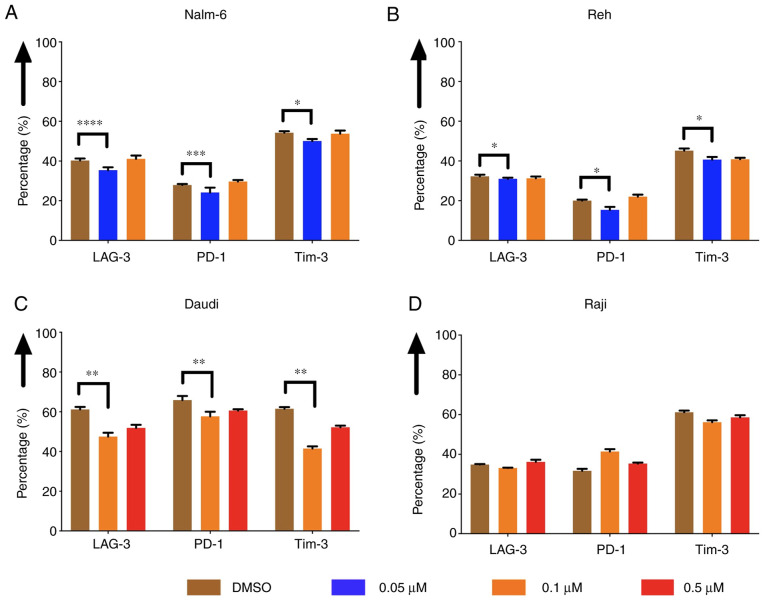
The expression of exhaustion markers of CAR T cells, such as LAG-3, PD-1 and Tim-3, was evaluated after co-culturing CAR T cells with pre-treated Nalm-6, Reh, Daudi and Raji cells. The expression was decreased when Nalm-6 and Reh had been pre-treated with eltanexor (0.05 µM) compared to DMSO [Nalm-6: LAG-3: 35.7±1.4 vs. 40.3±1.0% (P<0.0001), PD-1: 24.1±2.5 vs. 27.9±0.5% (P=0.0004), Tim-3: 50.1±1.0 vs. 54.3±0.8% (P=0.0225) (Fig. 7A); Reh: LAG-3: 31.0±0.6 vs. 32.3±0.8% (P=0.0335), PD-1: 15.4±1.5% vs. 20.0±0.5% (P=0.0317), Tim-3: 40.7±1.3 vs. 45.7±1.2% (P=0.0100) (Fig. 7B)]. Exhaustion marker expression on CAR T cells co-cultured with pre-treated Daudi cells (0.1 µM eltanexor) compared to DMSO was also decreased [LAG-3: 47.5±2.0 vs. 61.2±1.2% (P=0.0030), PD-1: 57.7±2.3 vs. 65.9±2.0% (P=0.1585), Tim-3: 41.5±1.1 vs. 61.6±0.8% (P=0.0060) (Fig. 7C)]. No difference in exhaustion marker expression was observed for CAR T cells co-cultured with pre-treated Raji cells (0.1 µM) (Fig. 7D). The decrease in exhaustion markers was not observed when CAR T cells were cultivated simultaneously with Daudi cells and eltanexor (Fig. S2).
Figure 7.
Expression of exhaustion markers of CAR T cells after co-culturing CAR T cells with tumor cells pre-treated with the SINE compound eltanexor. Nalm-6 (A) Reh (B) Daudi (C) and Raji (D) cells were cultivated with extanexor at different concentrations (0.05 µM: blue, 0.1 µM: Orange, 0.5 µM: Red) or with DMSO as control (brown) for 24 h. After washing with medium, CAR T cells were added, and co-culturing was performed for 5 days. Expression of exhaustion markers LAG-3, PD-1 and Tim-3 on CAR T cells decreased when tumor cells had been pre-treated with eltanexor compared to DMSO control. No significant alteration of the expression of exhaustion markers was observed when CAR T cells were co-cultured with Raji cells previously pre-treated with eltanexor. (D) Experiments were performed in triplicate. Results are represented as mean ± standard deviation (SD). *P<0.05, **P<0.005, ***P<0.0005, ****P<0.00005 indicate statistical significance.
Discussion
In patients with lymphoid malignancies CAR T cells have mediated high response rates. However, relapses and resistance after treatment with CAR T-cell therapy (10,45) constitute a challenge; to address this, several approaches are under investigation. To improve persistence and efficacy of CAR T cells, CAR T-cell production can be enhanced: For example, the PI3Kδ inhibitor idelalisib rendered enhanced in vivo function to CAR T cells that were manufactured from T-lymphocytes of CLL patients (42), a starting T-cell population with limited CAR T-cell responses due to functional characteristics of terminally differentiated lymphocytes (46). In addition, armored CAR T cells have been developed. Due to additional genetic modifications, these advanced CAR T cells intrinsically express additional costimulatory ligands or cytokines to augment CAR T-cell response (47). Furthermore, approaches that combine different mechanisms to target malignancies are of considerable interest in order to prevent escape of malignant cells from CAR T-cell treatment. Combination of CAR T cells with PD-1/PD-L1 inhibitors has been shown to enhance CAR T-cell efficacy and improve the clinical outcome of treated patients (48,49). CAR T cells combined with reactive oxygen species (ROS) accelerators were able to overcome tumor microenvironment-mediated treatment resistance (43). In combination with ibrutinib, CAR T-cell proliferation and antitumor efficacy in a human xenograft model were enhanced (50) while occurrence of CAR T-cell toxicity, i.e., cytokine release syndrome (CRS), was reduced (51). Recent clinical studies confirmed these data rendering superior clinical responses to CLL patients treated concomitantly with ibrutinib and CAR T cells (52).
Due to their general anti-malignant effect, SINEs constitute interesting combination partners for CAR T-cell therapy. XPO1 promotes cell deregulation exporting TSPs involved in apoptotic-inhibition from the nucleus (53) and SINEs can disrupt this process and regain tumor control (33,35,53). In this study, we evaluated the potential of SINEs in combination with third-generation CD19.CAR T cells.
The approved SINE compound selinexor as well as the second-generation SINE eltanexor mediated robust in vitro growth-inhibition of CD19-positive tumor cell lines. These data are in accordance with previous reports demonstrating that selinexor at a concentration up to 0.22 µM induced apoptosis in isolated MM cells (27). Eltanexor has been shown to mediate apoptosis in primary CLL cells and significantly inhibited proliferation of DLBCL cell lines (54). Moreover, eltanexor at a concentration of 0.15 µM has shown to induce apoptosis in AML cell lines but has displayed a better tolerability when compared to selinexor (55). With regards to the superior toxicity profile of eltanexor over selinexor as demonstrated also by others (39), we performed experiments addressing the combinatorial approach of SINEs and CAR T cells with eltanexor.
Our data demonstrate that sensitivity of tumor cells to SINEs correlated with the XPO1 protein levels in Nalm-6 and Reh cells. Besides confirming the anti-tumor efficacy of SINEs on malignant cells, the impact of SINEs on T cells, i.e., also CAR T cells, was assessed. It is known that in T cells XPO1-inhibition affects transcription factors that are crucial for T-cell functionality, e.g., NFATc1, p100 and p65 (subunits of NF-κB), cIAP1, stat1 and STAT3 (36,55,56). We confirmed this by observing that eltanexor decreased the levels of phosphorylated STAT3. The reduction of phosphorylated STAT3 in the cytoplasm with unaltered levels of total STAT3 suggests that retention of STAT3 within the nucleus may impair anti-tumor function of CAR T cells. This is in line with previous findings that demonstrated that the XPO1 inhibitor leptomycin B decreased the levels of phosphorylated STAT3 in the cytoplasm by limiting the transport through the nuclear membrane and accumulating the inactive STAT3 conformation within the nucleus (57). In addition, CLL patients achieving a complete response after CAR T-cell treatment had higher activation levels of the IL-6/STAT3 pathway when compared to non-responding patients, suggesting that decrease of phosphorylated-STAT3 is associated with poor clinical outcomes (58). Moreover, a novel gene-edited CAR containing a JAK-STAT3 signaling domain mediated superior anti-tumor effects (59).
Despite identifying STAT3 as a relevant SINE target, further studies extending the analysis to other relevant proteins and transcription factors in CAR T cells are required to define CAR T-cell impairment by SINEs. Our study is further limited by the fact that the effect of SINEs on CAR T cells was only assessed under artificial two-dimensional cell culture conditions, which do not reflect the dynamic activity of SINEs in tumor cells and in CAR T cells. Further evaluations in more complex culture conditions are required to clarify the mechanisms of the combination of SINEs and CAR T cells.
With regard to a potential combinatory approach, optimal synergistic effects of SINEs with CAR T cells should render effective inhibition of target tumor cells without affecting CAR T cells. Given that in this study SINEs impaired CAR T-cell function already at low concentrations, concomitant administration of SINEs and CAR T cells does not seem advisable. However, applying a pre-treatment strategy to protect the CAR T cells from SINEs seems to be promising. In fact, when used sequentially, pre-treatment with eltanexor mediated enhanced anti-tumor cytotoxicity of CAR T cells. The increase in secretion of cytokines and the decrease in expression of exhaustion markers on CAR T cells were consistent with improved cytotoxicity, which may-at least partially-explain the mechanism of enhancement.
According to our data, Reh and Nalm-6 were more sensitive than NHL cells Daudi and Raji to SINE treatment. Accordingly, we chose 0.05 and 0.1 µM as SINE concentrations for the ALL cell group (Reh and Nalm-6 cells), and higher concentrations of 0.1 and 0.5 µM to treat the NHL group (Daudi and Raji cells). In fact, in both groups a lower SINE concentration (0.05 µM for ALL; 0.1 µM for NHL) was more effective, increasing CAR T-cell cytotoxicity and enhancing CAR T-cell cytokine release. Consequently, pre-sensitizing tumor cells with SINEs may display a window of effect-concentration, whereby higher SINE concentrations may be associated with severe damage and killing of tumor cells resulting in loss of targets for CAR T cells and consequently decreased cytokine release. In contrast, lower SINE concentrations may pre-sensitize the tumor cells only (without completely killing them), preparing them for treatment with CAR T cells. Taken together, this finding is clinically promising and supports the combination approach of SINEs and CAR T cells as a low dose of SINEs displaying a favourable toxicity profile may be sufficient to enhance CAR T-cell function.
In summary, this study has focused on the intrinsic toxicity of SINEs against malignant cells. The toxicity of SINEs, however, also affected CAR T cells and significantly impaired their function, thereby limiting the potential for the combination and concomitant application of SINEs and CAR T cells. Nonetheless, pre-sensitizing tumor cells with eltanexor was shown to be an effective strategy to improve anti-malignant effects. Therefore, sequential use of SINEs and CAR T cells is a potential option to improve the efficacy of CAR T-cell treatment and should be addressed in further trials.
Supplementary Material
Acknowledgements
We thank Amy Publicover for editing the text. We are grateful to Ulrike Gern and Stefanie Mechler as technicians supporting this study.
Funding Statement
MLS was supported by the Olympia Morata Program of the Medical Faculty of Heidelberg. SW was supported by the China Scholarship Council.
Funding
MLS was supported by the Olympia Morata Program of the Medical Faculty of Heidelberg. SW was supported by the China Scholarship Council.
Availability of data and materials
The datasets used and/or analyzed within this study are available from the corresponding author on reasonable request. Original flow cytometry analysis data are displayed within the supplementary material (Figs. S3–S12).
Authors' contributions
SW, MLS, MS and LS designed the study; SW and HY performed the experiments; SW analyzed the data and wrote the primary manuscript; MLS, LS, TS and MS revised the manuscript critically for important intellectual content; SW, LW, MLS, LS, BN, WG, SS, MN, CK, AS and CMT discussed and contributed to the experimental design. SW and MLS are responsible for confirming the authenticity of all the raw data. All authors reviewed the manuscript. All authors approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
MS received funding for collaborative research from Apogenix, Hexal and Novartis, travel grants from Hexal and Kite, financial support for educational activities and conferences from Bluebird Bio, Kite and Novartis, is an advisory board member for MSD and (co-)PI of clinical trials of MSD, GSK, Kite and BMS, as well as co-founder and shareholder of TolerogenixX, Ltd. AS received travel grants from Hexal and Jazz Pharmaceuticals, research grant from Therakos/Mallinckrodt and is co-founder of TolerogenixX, Ltd. AS is a part-time employee of TolerogenixX, Ltd. LS is a full-time employee of Takeda (current address: Oncology Business Unit, Takeda Pharma Vertrieb GmbH & Co. KG, Berlin, Germany). LW is a full-time employee of TolerogenixX, Ltd. CMT: Bayer AG (research support); Pfizer, Janssen-Cilag GmbH (advisory board member). Pfizer, Daiichi Sankyo, BiolineRx (grants and/or provision of investigational medicinal products). SW, BN, WG, SS, MN, HY, CK, TS and MLS have no conflict of interest to declare.
References
- 1.Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, Ganguly N. CAR T cell therapy: A new era for cancer treatment (Review) Oncol Rep. 2019;42:2183–2195. doi: 10.3892/or.2019.7335. [DOI] [PubMed] [Google Scholar]
- 2.Ma CC, Wang ZL, Xu T, He ZY, Wie YQ. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol Adv. 2020;40:107502. doi: 10.1016/j.biotechadv.2019.107502. [DOI] [PubMed] [Google Scholar]
- 3.Schubert ML, Hückelhoven A, Hoffmann JM, Schmitt A, Wuchter P, Sellner L, Hofmann S, Ho AD, Dreger P, Schmitt M. Chimeric antigen receptor T cell therapy targeting CD19-positive leukemia and lymphoma in the context of stem cell transplantation. Hum Gene Ther. 2016;27:758–771. doi: 10.1089/hum.2016.097. [DOI] [PubMed] [Google Scholar]
- 4.Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, Riviere I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, Wood B, Lozanski A, Byrd JC, Heimfeld S, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35:3010–3020. doi: 10.1200/JCO.2017.72.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grupp S, Hu ZH, Zhang Y, Keating A, Pulsipher MA, Philips C, Margossian SP, Rosenthal J, Salzberg D, Schiff DE, et al. Tisagenlecleucel Chimeric Antigen Receptor (CAR) T-Cell Therapy for Relapsed/Refractory Children and young adults with Acute Lymphoblastic Leukemia (ALL): Real World Experience from the Center for International Blood and Marrow Transplant Research (CIBMTR) and Cellular Therapy (CT) Registry. Blood. 2019;134:2619. doi: 10.1182/blood-2019-129279. [DOI] [Google Scholar]
- 14.Jaglowski S, Hu ZH, Zhang Y, Kamdar M, Ghosh M, Lulla P, Sasine J, Perales MA, Hematti P, Nikiforow S, et al. Tisagenlecleucel Chimeric Antigen Receptor (CAR) T-Cell Therapy for Adults with Diffuse Large B-Cell Lymphoma (DLBCL): Real World Experience from the Center for International Blood & Marrow Transplant Research (CIBMTR) Cellular Therapy (CT) Registry. Blood. 2019;134:766. doi: 10.1182/blood-2019-130983. [DOI] [Google Scholar]
- 15.Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, Dahiya S, Lunning M, Lekakis L, Reagan P, et al. Standard-of-Care Axicabtagene Ciloleucel for relapsed or refractory large B-cell lymphoma: Results From the US Lymphoma CAR T consortium. J Clin Oncol. 2020;38:3119–3128. doi: 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer J, Paret C, El Malki K, Alt F, Wingerter A, Neu MA, Kron B, Russo A, Lehmann N, Roth L, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40:187–195. doi: 10.1097/CJI.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–385. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook A, Bono F, Jinek M, Conti E. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem. 2007;76:647–671. doi: 10.1146/annurev.biochem.76.052705.161529. [DOI] [PubMed] [Google Scholar]
- 22.Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen P, Sun YJ, Tang LN, Zheng SE. The expression of CRM1 is associated with prognosis in human osteosarcoma. Oncol Rep. 2009;21:229–235. [PubMed] [Google Scholar]
- 23.van der Watt PJ, Zemanay W, Govender D, Hendricks DT, Parker MI, Leaner VD. Elevated expression of the nuclear export protein, CRM1 (exportin 1), associates with human oesophageal squamous cell carcinoma. Oncol Rep. 2014;32:730–738. doi: 10.3892/or.2014.3231. [DOI] [PubMed] [Google Scholar]
- 24.Noske A, Weichert W, Niesporek S, Roske A, Buckendahl AC, Koch I, Sehouli J, Dietel M, Denkert C. Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer. 2008;112:1733–1743. doi: 10.1002/cncr.23354. [DOI] [PubMed] [Google Scholar]
- 25.Shen A, Wang Y, Zhao Y, Zou L, Sun L, Cheng C. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery. 2009;65:153–160. doi: 10.1227/01.NEU.0000348550.47441.4B. [DOI] [PubMed] [Google Scholar]
- 26.van der Watt PJ, Maske CP, Hendricks DT, Parker MI, Denny L, Govender D, Birrer MJ, Leaner VD. The Karyopherin proteins, Crm1 and Karyopherin beta1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation. Int J Cancer. 2009;124:1829–1840. doi: 10.1002/ijc.24146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, Tannenbaum D, Cagnetta A, Reagan M, Munshi AA, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: Molecular mechanisms and therapeutic implications. Leukemia. 2014;28:155–165. doi: 10.1038/leu.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima K, Kornblau SM, Ruvolo V, Dilip A, Duvvuri S, Davis RE, Zhang M, Wang Z, Coombes KR, Zhang N, et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood. 2013;121:4166–4174. doi: 10.1182/blood-2012-08-447581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo B, Huang L, Gu Y, Li C, Lu H, Chen G, Peng Z, Feng Z. Expression of exportin-1 in diffuse large B-cell lymphoma: Immunohistochemistry and TCGA analyses. Int J Clin Exp Pathol. 2018;11:5547–5560. [PMC free article] [PubMed] [Google Scholar]
- 30.Lapalombella R, Sun Q, Williams K, Tangeman L, Jha S, Zhong Y, Goettl V, Mahoney E, Berglund C, Gupta S, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120:4621–4634. doi: 10.1182/blood-2012-05-429506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang K, Wang M, Tamayo AT, Shacham S, Kauffman M, Lee J, Zhang L, Ou Z, Li C, Sun L, et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Exp Hematol. 2013;41:67–78.e4. doi: 10.1016/j.exphem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Bahlis NJ, Sutherland H, White D, Sebag M, Lentzsch S, Kotb R, Venner CP, Gasparetto C, Del Col A, Neri P, et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood. 2018;132:2546–2554. doi: 10.1182/blood-2018-06-858852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vercruysse T, De Bie J, Neggers JE, Jacquemyn M, Vanstreels E, Schmid-Burgk JL, Hornung V, Baloglu E, Landesman Y, Senapedis W, et al. The second-generation exportin-1 inhibitor KPT-8602 demonstrates potent activity against acute lymphoblastic leukemia. Clin Cancer Res. 2017;23:2528–2541. doi: 10.1158/1078-0432.CCR-16-1580. [DOI] [PubMed] [Google Scholar]
- 34.Ming M, Wu W, Xie B, Sukhanova M, Wang W, Kadri S, Sharma S, Lee J, Shacham S, Landesman Y, et al. XPO1 inhibitor selinexor overcomes intrinsic ibrutinib resistance in mantle cell lymphoma via nuclear retention of IκB. Mol Cancer Ther. 2018;17:2564–2574. doi: 10.1158/1535-7163.MCT-17-0789-ATR. [DOI] [PubMed] [Google Scholar]
- 35.Kuruvilla J, Savona M, Baz R, Mau-Sorensen PM, Gabrail N, Garzon R, Stone R, Wang M, Savoie L, Martin P, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood. 2017;129:3175–3183. doi: 10.1182/blood-2016-11-750174. [DOI] [PubMed] [Google Scholar]
- 36.Gravina GL, Senapedis W, McCauley D, Baloglu E, Shacham S, Festuccia C. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol. 2014;7:85. doi: 10.1186/s13045-014-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, Moreau P, Dingli D, Cole C, Lonial S, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381:727–738. doi: 10.1056/NEJMoa1903455. [DOI] [PubMed] [Google Scholar]
- 38.Machlus KR, Wu SK, Vijey P, Soussou TS, Liu ZJ, Shacham E, Unger TJ, Kashyap T, Klebanov B, Sola-Visner M, et al. Selinexor-induced thrombocytopenia results from inhibition of thrombopoietin signaling in early megakaryopoiesis. Blood. 2017;130:1132–1143. doi: 10.1182/blood-2016-11-752840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etchin J, Berezovskaya A, Conway AS, Galinsky IA, Stone RM, Baloglu E, Senapedis W, Landesman Y, Kauffman M, Shacham S, et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia. 2017;31:143–150. doi: 10.1038/leu.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schubert ML, Schmitt A, Sellner L, Neuber B, Kunz J, Wuchter P, Kunz A, Gern U, Michels B, Hofmann S, et al. Treatment of patients with relapsed or refractory CD19+ lymphoid disease with T lymphocytes transduced by RV-SFG.CD19.CD28.4-1BBzeta retroviral vector: A unicentre phase I/II clinical trial protocol. BMJ Open. 2019;9:e026644. doi: 10.1136/bmjopen-2018-026644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann JM, Schubert ML, Wang L, Huckelhoven A, Sellner L, Stock S, Schmitt A, Kleist C, Gern U, Loskog A, et al. Differences in expansion potential of naive chimeric antigen receptor T cells from healthy donors and untreated chronic lymphocytic leukemia patients. Front Immunol. 2018;8:1956. doi: 10.3389/fimmu.2017.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stock S, Ubelhart R, Schubert ML, Fan F, He B, Hoffmann JM, Wang L, Wang S, Gong W, Neuber B, et al. Idelalisib for optimized CD19-specific chimeric antigen receptor T cells in chronic lymphocytic leukemia patients. Int J Cancer. 2019;145:1312–1324. doi: 10.1002/ijc.32201. [DOI] [PubMed] [Google Scholar]
- 43.Yoo HJ, Liu Y, Wang L, Schubert ML, Hoffmann JM, Wang S, Neuber B, Huckelhoven-Krauss A, Gern U, Schmitt A, et al. Tumor-specific reactive oxygen species accelerators improve chimeric antigen receptor t cell therapy in B cell malignancies. Int J Mol Sci. 2019;20:2469. doi: 10.3390/ijms20102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Gong W, Wang S, Neuber B, Sellner L, Schubert ML, Huckelhoven-Krauss A, Kunz A, Gern U, Michels B, et al. Improvement of in vitro potency assays by a resting step for clinical-grade chimeric antigen receptor engineered T cells. Cytotherapy. 2019;21:566–578. doi: 10.1016/j.jcyt.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-hodgkin lymphoma. Lancet. 2017;390:298–310. doi: 10.1016/S0140-6736(16)32407-2. [DOI] [PubMed] [Google Scholar]
- 46.Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, Ramsay AG, Gribben JG. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schubert ML, Hoffmann JM, Dreger P, Muller-Tidow C, Schmitt M. Chimeric antigen receptor transduced T cells: Tuning up for the next generation. Int J Cancer. 2018;142:1738–1747. doi: 10.1002/ijc.31147. [DOI] [PubMed] [Google Scholar]
- 48.Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, Lewis ID, Brenner MK, Brown MP. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24:1135–1149. doi: 10.1038/mt.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Y, Lu W, Sun R, Jin X, Cheng L, He X, Wang L, Yuan T, Lyu C, Zhao M. Anti-CD19 chimeric antigen receptor T cells in combination with nivolumab are safe and effective against relapsed/refractory B-cell Non-Hodgkin lymphoma. Front Oncol. 2019;9:767. doi: 10.3389/fonc.2019.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, Lacey SF, Melenhorst JJ, McGettigan SE, Cook DR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127:1117–1127. doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruella M, Kenderian SS, Shestova O, Klichinsky M, Melenhorst JJ, Wasik MA, Lacey SF, June CH, Gill S. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-cd19 chimeric antigen receptor t cells for b-cell neoplasms. Leukemia. 2017;31:246–248. doi: 10.1038/leu.2016.262. [DOI] [PubMed] [Google Scholar]
- 52.Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, Yeung CCS, Sheih A, Pender BS, Hawkins RM, et al. Feasibility and efficacy of CD19-targeted CAR-T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135:1650–1660. doi: 10.1182/blood.2019002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan DS, Bedard PL, Kuruvilla J, Siu LL, Razak AR. Promising SINEs for embargoing nuclear-cytoplasmic export as an anticancer strategy. Cancer Discov. 2014;4:527–537. doi: 10.1158/2159-8290.CD-13-1005. [DOI] [PubMed] [Google Scholar]
- 54.Hing ZA, Fung HY, Ranganathan P, Mitchell S, El-Gamal D, Woyach JA, Williams K, Goettl VM, Smith J, Yu X, et al. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia. 2016;30:2364–2372. doi: 10.1038/leu.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu D, Grishin NV, Chook YM. Nesdb: A database of NES-containing CRM1 cargoes. Mol Biol Cell. 2012;23:3673–3676. doi: 10.1091/mbc.e12-01-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyler PM, Servos MM, de Vries RC, Klebanov B, Kashyap T, Sacham S, Landesman Y, Dougan M, Dougan SK. Clinical dosing regimen of selinexor maintains normal immune homeostasis and T-cell effector function in mice: Implications for combination with immunotherapy. Mol Cancer Ther. 2017;16:428–439. doi: 10.1158/1535-7163.MCT-16-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhattacharya S, Schindler C. Regulation of STAT3 nuclear export. J Clin Invest. 2003;111:553–559. doi: 10.1172/JCI15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O'Connor RS, Hwang WT, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang CH, Saso K, Butler MO, Minden MD, Hirano N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018;24:352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed within this study are available from the corresponding author on reasonable request. Original flow cytometry analysis data are displayed within the supplementary material (Figs. S3–S12).