Abstract
Background
Pediatric anesthesiology has been greatly impacted by COVID‐19 in the delivery of care to patients and to the individual providers. With this study, we sought to survey pediatric centers and highlight the variations in care related to perioperative medicine during the COVID‐19 pandemic, including the availability of protective equipment, the practice of pediatric anesthesia, and economic impact.
Aim
The aim of the survey was to determine how COVID‐19 directly impacted pediatric anesthesia practices during the study period.
Methods
A survey concerning four major domains (testing, safety, clinical management/policy, economics) was developed. It was pilot tested for clarity and content by members of the Pediatric Anesthesia COVID‐19 Collaborative. The survey was administered by email to all Pediatric Anesthesia COVID‐19 Collaborative members on September 1, 2020. Respondents had six weeks to complete the survey and were instructed to answer the questions based on their institution's practice during September 1 ‐ October 13, 2020.
Results
Sixty‐three institutions (100% response rate) participated in the COVID‐19 Pediatric Anesthesia Survey. Forty‐one hospitals (65%) were from the United States, and 35% included other countries. N95 masks were available to anesthesia teams at 91% of institutions (n = 57) (95% CI: 80%–96%). COVID‐19 testing criteria of anesthesia staff and guidelines to return to work varied by institution. Structured simulation training aimed at improving COVID‐19 safety and patient care occurred at 62% of institutions (n = 39). Pediatric anesthesiologists were economically affected by a reduction in their employer benefits and restriction of travel due to employer imposed quarantine regulations.
Conclusion
Our data indicate that the COVID‐19 pandemic has impacted the testing, safety, clinical management, and economics of pediatric anesthesia practice. Further investigation into the long‐term consequences for the specialty is indicated.
Keywords: COVID‐19, hospital economics, pediatric anesthesia, personal protective equipment, preoperative testing, simulation
Clinical Implications
What is already known?
The COVID‐19 global pandemic has upended traditional hospital policies regarding preoperative testing, personal protective equipment, staffing, and visitation. No data exist on how operative care has changed as a result of the pandemic or its impact on perioperative patients and providers.
What this article adds
Responses showed COVID‐19 had variable effects based on geographic location and institution. It has strained healthcare resources, and drastically changed the environment in which pediatric anesthesiologists practice.
1. INTRODUCTION
In December 2019 a novel coronavirus, SARS‐CoV‐2 and its resulting disease COVID‐19, were first reported in China. 1 , 4 By March 2020, COVID‐19 was declared a worldwide public health crisis. As of December 2020, the number of global cases is 76 704 426 and the number of global deaths is 1 691 198 and climbing. 2 Currently, the overall rate of child COVID‐19 cases in the United States is 3336 cases per 100 000 children in the population. 3 In addition, the disease affects children and those who take care of children in different ways. Initial studies from China indicate that generally children experience less severe disease, and only 5.3% become severely ill and 0.6% critically ill. 4 Perhaps because of the more serious disease pattern among adults, information published on children is limited and still evolving. 5 , 6
This novel coronavirus has disrupted the access, delivery, and economics of healthcare. The American Hospital Association estimates an average loss of $50.7 billion per month for hospitals and health care systems from March 1 to June 30, 2020. 7 Anesthesiology, including pediatric anesthesiology, has been greatly impacted by COVID‐19, not just in the delivery of care to patients but for the individual anesthesiology providers as well. Much of the current pediatric anesthesia COVID‐19 literature is focused on how to care for children in the perioperative setting, including methods for the resumption of elective outpatient surgery through preoperative testing. 8 , 9 , 10
COVID‐19 directly impacted anesthesiologists because their own health is at risk when caring for infected patients. In addition, the pandemic increased emotional stress and created the possibility of adverse financial impact. Pediatric anesthesiologists, especially in the United States, were economically affected by a reduction in their employer benefits and restriction of important personal or family related travel due to employer imposed quarantine regulations. Anesthesiologists, like many other healthcare providers, struggled to obtain adequate personal protective equipment and adequate COVID‐19 testing for patients and themselves. Adult hospitals and intensive care units have been at times overwhelmed with patients, and some pediatric anesthesiologists were deployed to assist. The pandemic's impact is far reaching on pediatric anesthesia education and training programs as well. In a recent report, pediatric anesthesiology fellows reported increased stress about contracting COVID‐19 at work, finding a job after graduation, and frustration with modified didactics. 11 Disease exposure and infections are also having adverse effects on perioperative workforce. 12
Internationally, pediatric anesthesiologists have seen a decrease of 10%–15% or more in elective case volumes during the height of the pandemic due to the fact that many hospitals suspended elective cases early in March and April 2020. 13 Since COVID‐19 emerged, national and local public health policies have changed several times resulting in mixed messages on appropriate personal protective equipment, testing strategies, and quarantine requirements. The pandemic resulted in halting elective surgical procedures in many locations for a period of time, and in many cases employers changed compensation packages resulting in economic uncertainty for many healthcare providers.
The aim of this survey was to determine how the COVID‐19 pandemic has impacted pediatric anesthesia departments and practice. The survey questions were structured around 4 domains including healthcare delivery, personal protective equipment access and policies, policies for COVID‐19 testing of patients and personnel, and economics. We sought to determine how COVID‐19 has affected the field of pediatric anesthesia over a large geographical range, and how pediatric anesthesiologists are transforming their practices. This information can help guide institutions to understand the details of the pandemic's impact and develop policies and procedures to keep their patients and anesthesia providers safe. Lessons learned from the operational challenges posed by COVID‐19 should be used to inform preparation for similar challenges in the future.
2. METHODS
Institutional Review Board (IRB) exemption was obtained from Boston Children's Hospital. A prospective survey questionnaire was developed by the study authors (supplemental material 1 & 2) and pilot tested for clarity and content by members of the Pediatric Anesthesia COVID‐19 Collaborative (PEACOC). Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed. All 63 member institutions were included. There were no exclusion criteria. The authors sent out an email link to all members of the Pediatric Anesthesia COVID‐19 Collaborative on September 1, 2020. Survey data were collected and managed with Research Electronic Data Capture (RedCap) software. If an institution had several anesthesiologists as members of the Pediatric Anesthesia COVID‐19 Collaborative, they were instructed to select a representative with knowledge of their institution's COVID‐19 policies to respond. Respondents had 6 weeks to complete the survey, and reminder emails were sent at the end of the time period. The survey closed on October 13, 2020. Respondents were instructed to answer the questions based on the current situation at their institutions during the study period September 1‐October 13, 2020. Individual institution data were fully de‐identified, so study authors knew only that a member from the corresponding institution had completed the survey.
2.1. Statistical analysis
Descriptive analyses were performed for all data collected on all respondents. Categorical variables are presented as frequencies and percentages in the respondent cohort. For survey questions within conditional branching logic, denominators are presented to indicate the sizes of respondent subgroups. Binomial exact 95% confidence intervals (CI) are calculated and presented for proportions for key measurements to provide precision around the observed data. All statistical analyses were performed using Stata (version 16.0, StataCorp LLC.).
3. RESULTS
3.1. Demographics and characteristics of respondents
A total of 63 responses were collected for the COVID‐19 Pediatric Anesthesia Survey. Demographics of respondents are presented in Figure 1. Forty‐one respondents (65%) were from academic hospitals, 15 (24%) were from private institutions, and 16 (27%) were from public (government supported) institutions. Forty‐six percent of institutions (n = 29) had 250 or more hospital beds dedicated to the pediatric population, and 54% had less than 250 beds. Forty‐one hospitals (65%) were located in the United States and 35% respondents (n = 22) came from other countries across the globe.
FIGURE 1.
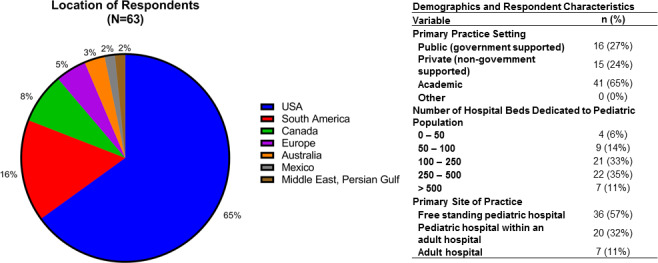
Description of respondent characteristics. The majority of respondents were at US hospitals, but many international locations were represented. Most respondents came from academic practice settings (65%) and free standing pediatric hospitals (57%). A range of hospital volumes were represented
3.2. COVID‐19 testing
Select COVID‐19 testing data are summarized in Figure 2. Patient polymerase chain reaction testing for COVID‐19 was required for elective surgery in 65% of hospitals (n = 41) and for urgent surgery in 56% of hospitals (n = 35). Thirteen percent of respondents (n = 8) reported that PCR testing was not used at their institution. Perioperative screening questionnaires were used at 92% of institutions (n = 58). Testing of anesthesia staff was performed routinely in 3% of institutions (n = 2), if having symptoms in 79% of institutions (n = 50), and if having known exposure in 49% of institutions (n = 31). In 67% of hospitals (n = 41), a negative COVID‐19 test was considered valid for 72 h or less.
FIGURE 2.
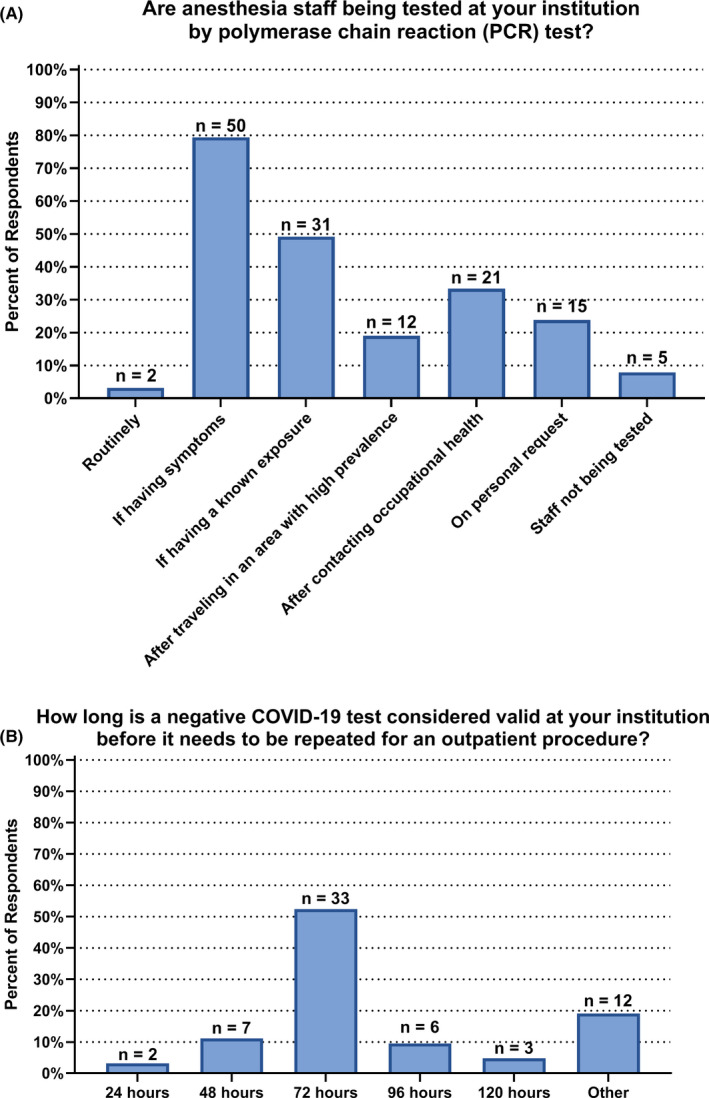
COVID‐19 Testing of pediatric anesthesia staff. A, At most institutions, pediatric anesthesia staff are receiving polymerase chain reaction (PCR) testing if having COVID‐19 symptoms, although there is variability across hospitals. B, Most often, a negative COVID‐19 test is considered valid for 72 h or less
3.3. Personal protective equipment
N95 masks were available to the anesthesia team at 91% of institutions (n = 57) (95% CI: 80%–96%). The most common personal protective equipment worn by anesthesiologists while caring for an untested, patient under investigation for COVID‐19, or COVID‐19 positive patient were N95 masks (89%; n = 56), face shields or goggles (86%; n = 54), hat/bonnet (79%; n = 50), and gloves (71%; n = 45). In the event of a shortage of personal protective equipment, 51% of institutions (n = 32) allowed anesthesiologists to use their own protective equipment (Figure 3). Among institutions that did not allow anesthesiologists to use their own personal protective equipment, cases were cancelled when personal protective equipment was not available in 82% (9/11), whereas 18% of hospitals (2/11) proceeded without appropriate personal protective equipment.
FIGURE 3.
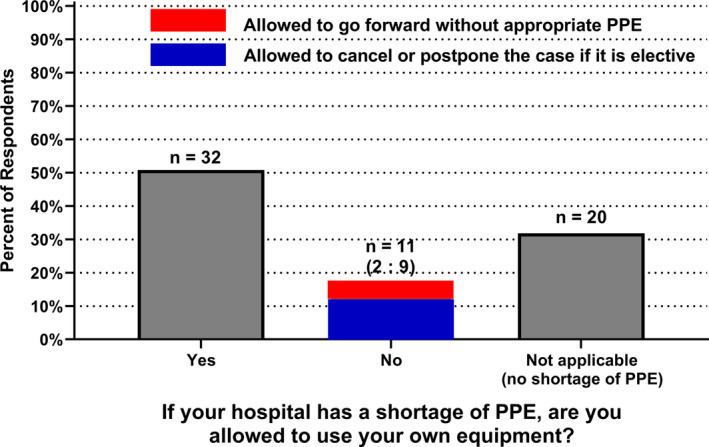
Personal protective equipment (PPE) during a shortage. Among the 43 respondents who had experienced PPE shortages at their institutions, 32 reported that the anesthesia staff was allowed to use their own PPE equipment during a shortage. In the 11 institutions where utilization of own PPE is not allowed, 2 allow going forward without appropriate PPE and 9 allow to cancel or postpone the case if it is elective
3.4. Clinical management
Seventy‐one percent of hospitals (n = 45) had airway barrier methods utilized when intubating a patient under investigation for COVID‐19 or COVID‐19 positive patient. In 38 institutions of these 45 (84%), it was at the discretion of the anesthesiologist in which age group airway barriers were utilized. Parents were allowed to be present during induction of general anesthesia in 16% of hospitals (n = 10). Parental presence was determined on a case‐by‐case basis in 25% of hospitals (n = 16). Only 6 hospitals (10%) were testing parents of patients. The patient's COVID‐19 status impacted the parental visitation policy in 73% of centers (n = 46), among which 78% (n = 36/46) allowed only one parent/legal guardian per patient to visit.
3.5. Impact on pediatric anesthesia staff
Figure 4 provides a summary of the impact of COVID‐19 on pediatric anesthesia staff. Disproportional fatigue due to COVID‐19 was reported in 65% of institutions (n = 41), and 10% of respondents (n = 6 out of 63) believed that medical errors increased in their institution during the pandemic. The guidelines for returning to work after testing positive for COVID‐19 varied by hospital 37% (n = 23) dependent on symptom resolution, 41% (n = 26) dependent on fixed number of days after symptoms started, 19% (n = 12) depended on repeat negative COVID‐19 test, 33% (n = 21) were decided by occupational health on a case‐by‐case basis. Concerning pay and benefits, incentive pay was negatively impacted at 46% of institutions (n = 29) (95% CI: 33%–59%) during the pandemic, vacation time was reduced at 27% of hospitals (n = 17) (95% CI: 17%–40%), and personal travel restrictions were implemented at 62% of hospitals (n = 39) (95% CI: 49%–74%). Sixty‐eight percent (n = 43) (95% CI: 55%–79%) of respondents indicated that staff were not given the choice regarding working with COVID‐19 positive patients (Figure 4A), whereas 32% of staff (n = 20) were excluded for reasons listed in Figure 4B.
FIGURE 4.
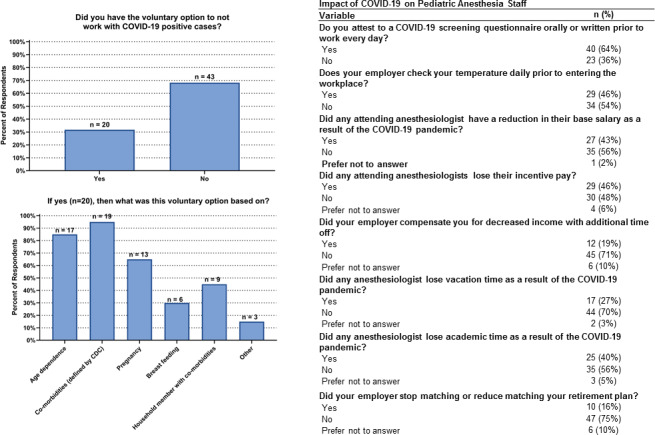
Impact of COVID‐19 on pediatric anesthesia staff. Twenty respondents reported having the voluntary option to not work with COVID‐19 positive cases, and this was most often based on age, comorbidities and pregnancy of the pediatric anesthesia staff. Many workplace procedures and economic factors in pediatric anesthesia staff were impacted due to the COVID‐19 pandemic
3.6. Infrastructure
Utilization of negative pressure operating rooms was found in 44% of hospitals (n = 28). For a COVID‐19 positive patient, staff without personal protective equipment was allowed to enter the operating room following intubation without a waiting period in 16% of hospitals (n = 10), and at the discretion of the team leader in 13% of hospitals (n = 8).
3.7. Training of Staff
Among the 62% (n = 39) of hospitals offering structured simulation training, 100% (n = 63) reported that simulation training led to staff to feel better‐prepared managing COVID‐19 patients (95% CI: 91%–100%, Figure 5). During the care of a COVID‐19 positive patient or patient under investigation for COVID‐19 64% of institutions (n = 40) had a designated spotter available for donning and doffing of personal protective equipment, however, during the off‐hours this safety feature was only available in 35% of institutions (n = 14/40).
FIGURE 5.
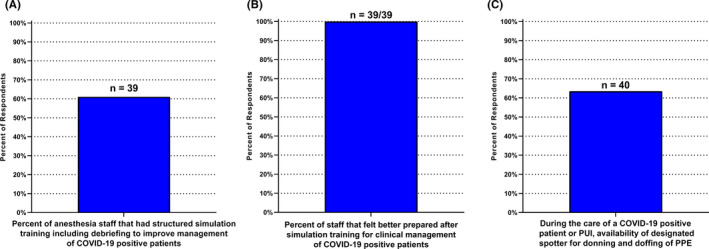
Training of pediatric anesthesia staff. A, At 39 among the 63 institutions, anesthesia staff had structured simulation training including debriefing to improve management of COVID‐19 positive patients. B, At all 39 of these institutions (100%; 95% CI: 91%–100%), staff felt better prepared for clinical management of COVID‐19 patients after simulation training. C, Availability of a designated spotter for donning and doffing of personal protective equipment (PPE) was available at 40/63 institutions. PUI = untested patient under investigation for COVID‐19
3.8. Policy and management
Respondents to the survey indicated that COVID‐19 had an impact on policy and management practices at their institutions. For example, 81% of respondents (n = 51) indicated that their hospitals followed recommendations regarding COVID‐19 set forth by their national anesthesia society for personal protective equipment use, and 67% of respondents (n = 42) indicated the same for patient COVID‐19 testing.
Eighteen percent of institutions (n = 11) mandated that paid days off were taken due to decreased case volume. These mandated days off were either randomly distributed (46%; n = 5/11), followed a planned distribution (46%; n = 5/11), or were evenly distributed (9%; n = 1/11). Since reopening, a returning volume of elective surgical cases has been observed at 36% of institutions (n = 4/11), however, not necessarily to prepandemic levels and these required extra shifts on weekends or extended work days at 25% of institutions (n = 1/4).
4. DISCUSSION
Our international survey has confirmed that the COVID‐19 pandemic brought forth widespread impact on pediatric anesthesiology. Numerous institutions required preprocedure patient COVID testing within 72 h of the procedure as outlined by the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation Statement on Perioperative Testing. 14 However, very few anesthesiology staff were being tested routinely (Figure 2) despite potential asymptomatic transmission, as pointed out by Stock et al who recommend frequent testing regimens of healthcare workers on a regular, multi‐modal basis to prevent spread within the workforce and to patients. 15 Hospitals relied more on questionnaires and daily attestations rather than routine testing of employees. In addition, most (57/63 respondents) institutions allowed parents to enter facilities without testing them for COVID‐19. A minority (25% of institutions) also allowed parents to enter the operating rooms for induction despite that they could be sources of asymptomatic spread. Variation of timing and testing could reflect local testing supplies, recommendations set forth by the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation, government and the subsequent local hospital policies, and healthcare system capacity. 13 , 16 , 17
Early in the COVID‐19 pandemic, there were reports of critical supply shortages of equipment and personal protective equipment in many facilities worldwide. 18 Compounding this problem was an increased demand and cost for personal protective equipment. 7 Our survey demonstrated that the vast majority (91%, 57 out of 63 responding institutions) of anesthesiologists had access to N95 masks. However, not all anesthesiologists had access to adequate personal protective equipment even though they participate in high‐risk aerosolizing procedures. This troubling finding was not in line with the recommendations set forth by the American Society of Anesthesiologists, Anesthesia Patient Safety Foundation and the World Health Organization. Unfortunately, our survey did not ascertain why this was not universal. However, our survey indicated that 22% of anesthesia providers left their practice because they felt unsafe taking care of COVID‐19 patients. Some institutions allowed employees to purchase and wear other approved personal protective equipment if it could not be supplied by the facility, as endorsed by the American Society of Anesthesiologists. 16 Among the 11 institutions where using one's own personal protective equipment was not allowed, anesthesiologists at 2 of these institutions had to proceed with elective cases in patients under investigation for COVID‐19 or COVID‐19 positive patients without personal protective equipment (Figure 3).
As the COVID‐19 pandemic surges, educational needs continue to require frequent reassessment to maintain safety and high clinical standards. Proper donning and doffing of personal protective equipment are important to prevent self‐contamination and spread of COVID‐19 to other staff and patients. Cook et al postulated that the lower than expected risk of occupational infection in our specialty might be due to the excellent attention to detail that most anesthesiologists have with respect to the use of personal protective equipment and robust airflow in the venues in which they work. 19 In addition, staff education on these practices, simulation training, and having spotters to ensure compliance would mitigate these risks as reviewed by Zucco et al. 17 Figure 5 highlights the results of our survey indicating that only 62% of responding institutions had simulation training for donning and doffing of personal protective equipment. Our survey found a reduction in spotter availability for donning and doffing of personal protective equipment during off‐hours. Furthermore, providers taking care of COVID‐19 positive patients needed to be proficient in new intubation techniques such as using barrier devices to prevent contamination. In our survey, 71% of institutions were using airway barrier methods. However, reports about experience with these novel protective barrier enclosures were controversial due to limitations on the ability to perform airway interventions and in containing aerosols during aerosol‐generating procedures. 20 , 21 Staff involved in formalized simulation training felt more prepared to deal with the cognitive load that accompanies taking care of COVID‐19 positive patients. As outlined by Gaba et al, simulation training has been found to advance patient safety and reduce the risk of medical errors. 22 In our survey disproportional fatigue due to COVID‐19 was reported in 65% of institutions, and 10% of respondents believed that medical errors increased in their institution during the pandemic. Our survey results emphasize how formal simulation training promotes a safety culture.
In March and April of 2020, many hospitals temporarily halted pediatric elective procedures, and the economic impact was tremendous. 23 Hospitals in the United States lost an average of $50.7 billion from March 1‐June 30 2020, according to the American Hospital Association. 7 Many hospitals in the United States depend on elective surgeries to stay financially solvent, and the sharp decline in revenue is exacerbated by increased costs associated with taking care of COVID‐19 positive patients. 7 The cessation of surgical elective cases led to a backlog of surgical elective cases. For healthcare systems to recover, many institutions required pediatric anesthesia providers to work extra hours upon resumption of normal clinical activity. Our survey results indicated that 25% of the anesthesia providers were required to work extra hours. The economic situation deteriorated for many Americans who were unemployed due to COVID‐19 including healthcare workers 24 and hospitals saw more uninsured patients as well. 7
During the pandemic, there were also changes to staffing roles to accommodate the need to care for COVID‐19 positive patients. Figure 4 demonstrates that 32% of survey respondents indicated that they could voluntarily elect to not work with COVID‐19 patients. Reasons to be excluded from working with COVID‐19 patients included the anesthesia provider's age, medical comorbidities, pregnancy, breastfeeding, and household members with comorbidities.
Our data indicate pediatric anesthesiology faculty also saw a reduction in their salary, incentive pay, decreased retirement matching and loss of nonclinical time during the pandemic. As stated by Krukowski et al, the pandemic disrupted academic productivity in many areas of science, technology, and medicine. 25 Evidence shows that trainees have been affected similarly, and pediatric anesthesia fellows reported anxiety over contracting COVID‐19 and securing employment after graduation in an uncertain job market. 11
Our study has several limitations. Although not a random sample, the study design is prospective, and consequently recall bias was not a limiting factor. Due to the novelty of COVID‐19, national and institutional guidelines have been revised to reflect evolving medical knowledge, and healthcare staffing challenges have developed. Nevertheless, the results of this study will help inform clinical decision‐making. Our survey and results represent a snapshot (September‐October 2020) in an ever‐changing healthcare environment affected by COVID‐19. However, it provides temporally relevant information as medical knowledge regarding the COVID‐19 pandemic evolves and requires reassessment.
An aspect of selection bias may be built into the survey as well. Our respondents were all members of an international pediatric anesthesiology research collaborative, and therefore anesthesiologists not part of the collaborative may have answered differently. Membership into the Pediatric Anesthesia COVID‐19 Collaborative arose from individuals actively participating in other research registries (Pediatric Craniofacial Collaborative Group, Difficult Airway Registry) that were established through the Society of Pediatric Anesthesia. It was done mainly through this mechanism which may have caused inadvertent selection bias. It is a voluntary professional group of pediatric anesthesiologists interested in how COVID‐19 is affecting our specialty. Membership is free and requires interest and availability to meet over Zoom video conferencing. However, given the heterogeneity of pediatric anesthesiology practices, we feel our sample does have validity because it is an international survey with a high response rate representing hospitals ranging in size and type.
Our survey elucidates differences in personal protective equipment and COVID‐19 testing availability in institutional safety practices and economics that directly affect pediatric anesthesia practices and providers. The majority of hospitals followed national guidelines. As COVID‐19 vaccine development is completed and vaccination programs are implemented, new safety practices, and the results of our survey will be carried forward to confront new challenges ahead for pediatric anesthesiology. Careful consideration of safety practices and their implementation will help inform optimal decision‐making and patient management. Further investigation into the long‐term consequences of COVID‐19 on our specialty is warranted.
5. Pediatric Anesthesia COVID‐19 Collaborative: Collaborating Authors
Sindu Balakrishnan, Vipin Bansal, Angela Becerra Torres, Amy Beethe, Hubert A Benzon, Angelina Bhandari, Ashley Bocanegra, Dylan Bould, Melissa Brooks Peterson, Alyssa Brzenski, Veronica Busso, James G Cain, Myles Cassidy, Eric C Cheon, Surendrasingh Chhabada, Lynnie R Correll, Nicholas M Dalesio, Andrew Davidson, Courtney Derderian, Vipul Dhumak, Nicola Disma, Ajay D'Mello, Piedad Echeverry, Pavithra R Ellison, Thomas Erb, Angelica Fajardo, Ricardo J Falcon, Allison Fernandez, Brian Frugoni, Javier García, Olga Lucía Giraldo, Chris D Glover, Jessica Goeller, Susan M Goobie, Ingrid Gooch, Lina Maria Granados, Anastasia Grivoyannis, Velu Guruswamy, Emily Hesselink, Jill Hobbs, Agnes Hunyady, Ranu Jain, Lydia Jorge‐Reynolds, Meredith A Kato, Michael R King, Jamie Kitzman, Jeffrey Koh, Andy Lester, Amanda Lorinc, Constanza Lozano, Katherine Manupipatpong, Clyde Matava, Duncan McLuckie, Kanwal Merchant, Heather Mitzel Levy, Bridget L Muldowney, Julian Andres Navarro, Jonathon Nelson, Amish Patel, Roshan Patel, Niroop Ravula, Desigen Reddy, Srijaya K Reddy, Megan Rodgers McCormick, Remigio Remi Roque, David Rosen, Elizabeth Rossmann Beel, Leelach Rothschild, Lina Sarmiento, Anna Shadrina, Robert Shaw, Michelle Sheth, Allan F Simpao, Neeta Singh, Timothy E Smith, Codruta Soneru, Claire Soria, Peter Szmuk, Brad M Taicher, Gee Mei Tan, Howard Teng, Edala Thejovathi, Nathaniel Tighe, Simon Tom, Alexander Trujillo, Susan R Vishneski, Juan Pablo Vivas, Adam Von Samek, Britta S von Ungern‐Sternberg, Simon Whyte, Robert T Wilder
6. Pediatric Anesthesia COVID‐19 Collaborative: Participating Institutions
Alaska Pacific Northwestern University, USA Alberta Health Services (AHS) University of Calgary ‐ Alberta Children's Hospital, Canada Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine, IL, USA Baylor College of Medicine (BCM), Texas Children’s Hospital, Houston, TX, USA Boston Children's Hospital, Harvard Medical School, Boston, MA, USA Brenner Children's Hospital and Wake Forest School of Medicine, Winston‐Salem, North Carolina, USA British Columbia’s Children’s Hospital and University of British Columbia, Vancouver BC, Canada CHEO, Children's Hospital of Eastern Ontario, Canada Children's Hospital Colorado, University of Colorado, School of Medicine, Aurora, CO, USA Children's Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA Children's National Hospital, Washington, DC, USA CHOA Children's Healthcare of Atlanta, Emory University, GA, USA Cincinnati Children's Hospital Medical Center (CCHMC), Ohio, USA Cleveland Clinic, Cleveland, OH, USA Clinica de Marly, Bogotá, Colombia Clínica Imbanaco, Cali, Colombia Clinica Pediatrica de Colsanitas, Bogotá, Colombia Covenant Children's Hospital, Lubbock, TX, USA Doernbecher Children's Hospital, Oregon Health & Science University, Portland, OR, USA Driscoll Children’s Hospital, Corpus Christi, TX, USA Duke University Medical Center, Durham, North Carolina, USA Fundación Hospital Infantil Napoleon Franco Pareja, Cartagena, Colombia Golisano Childrens’ Hospital at the University of Rochester, Rochester, NY, USA Hospital for Sick Children,Toronto, Canada Hospital Infantil de Manizales, Manizales,Colombia Hospital Pediátrica La Misericordia, Bogotá,Colombia Hospital Universitario San Ignacio, Bogotá, Colombia Hospital Universitario San Vicente de Paúl, Medellín, Colombia Hospital Universitario Simon Bolivar, Bogotá, Colombia Instituto Roosevelt, Bogota, Colombia Istituto Giannina Gaslini, Genova, Italy Jackson Memorial Hospital, University of Miami, Miami, FL, USA Johns Hopkins All Children’s Hospital, Saint Petersburg, FL, USA Johns Hopkins Children's Center, The Johns Hopkins Hospital, Baltimore, MD, USA Mayo Clinic, Rochester, Minnesota, USA McMaster University, Hamilton, Ontario, Canada Monroe Carell Jr. Children's Hospital / Vanderbilt University Medical Center (VUMC), Nashville, Tennessee, USA Montefiore Medical Center, Bronx, Albert Einstein College of Medicine, NY, USA Mount Sinai Hospital, Moningside, West, Queens, USA MSKCC, Memorial Sloan Kettering Cancer Center, NY, USA National Institute of Pediatrics, Mexico City, Mexico Nationwide Children's, Columbus, The Ohio State University (OSU), OH, USA NYU Langone Health, New York City , NYU Grossman School of Medicine, NY, USA Omaha Children's Hospital & Medical Center; University of Nebraska Medical Center, Omaha, NE, USA Perth Children's Hospital and The University of Western Australia, Perth, Western Australia, Australia Royal Children's Hospital & Murdoch Children's Research Institute, University of Melbourne, Victoria, Australia Seattle Children’s Hospital, University of Washington School of Medicine, Seattle, WA, USA Sheffield Children's Hospital, UK Shriners Hospital for Children, Chicago, USA Sidra Medicine, Weil Cornell Medical College, Doha, Qatar Universitäts‐Kinderspital beider Basel (UKBB), Basel, Switzerland University Hospitals Rainbow Babies and Children’s Hospital, Cleveland, OH, USA University of Arkansas for Medical Sciences (UAMS), Arkansas Children's Hospital, Arkansas, USA University of California Davis, UC Davis Children's Hospital, Sacramento, CA, USA University of California, San Diego (UC San Diego), and Rady Children's Hospital, CA, USA University of Illinois at Chicago, Chicago, USA University of Kansas Medical Center, USA University of Mississippi Medical Center (UMMC) Jackson, MS, USA University of New Mexico (UNM), Albuquerque, New Mexico, USA University Texas Health Science (UTH) Houston, McGovern Medical School at UT Health, TX, USA University of Wisconsin‐Madison American Family Children’s Hospital, WI, USA University of Texas Southwestern Medical Center, Dallas, TX, Children's Health Medical Center, Dallas, TX, & Outcome Research Consortium, Cleveland, OH, USA West Virginia University School of Medicine (WVU), USA
DISCLOSURES
Bradford: honorarium for American Society of Anesthesiologists (ASA) Self‐Education and Evaluation (SEE) Program questions; Raman: equity in NDS Kalstars and Opus INFLUUNT and Face To Face, honorarium from Merck, honorarium from the American Society of Anesthesiologists (ASA) for Anesthesia Continuing Education (ACE) questions, Nationwide Children's Hospital Intramural grant; Fernandez, Meier, Soneru, Staffa, Zurakowski: none.
ETHICS
Boston Children's Hospital and Harvard Medical School IRB Institution exempt.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
Jocelyn Booth BSN, RN, CPN (Research Nurse) for building, distributing and providing the administrative support for the RedCap Survey with the technical support of Sheng Xian Huang, BS (Clinical Research Assistant) of the Department of Anesthesiology, Critical Care and Pain Medicine, BCH.
APPENDIX 1.
Sindu Balakrishnan, Vipin Bansal, Angela Becerra Torres, Amy Beethe, Hubert A Benzon, Angelina Bhandari, Ashley Bocanegra, Dylan Bould, Melissa Brooks Peterson, Alyssa Brzenski, Veronica Busso, James G Cain, Myles Cassidy, Eric C Cheon, Surendrasingh Chhabada, Lynnie R Correll, Nicholas M Dalesio, Andrew Davidson, Courtney Derderian, Vipul Dhumak, Nicola Disma, Ajay D'Mello, Piedad Echeverry, Pavithra R Ellison, Thomas Erb, Angelica Fajardo, Ricardo J Falcon, Allison Fernandez, Brian Frugoni, Javier García, Olga Lucía Giraldo, Chris D Glover, Jessica Goeller, Susan M Goobie, Ingrid Gooch, Lina Maria Granados, Anastasia Grivoyannis, Velu Guruswamy, Emily Hesselink, Jill Hobbs, Agnes Hunyady, Ranu Jain, Lydia Jorge‐Reynolds, Meredith A Kato, Michael R King, Jamie Kitzman, Jeffrey Koh, Andy Lester, Amanda Lorinc, Constanza Lozano, Katherine Manupipatpong, Clyde Matava, Duncan McLuckie, Kanwal Merchant, Heather Mitzel Levy, Bridget L Muldowney, Julian Andres Navarro, Jonathon Nelson, Amish Patel, Roshan Patel, Niroop Ravula, Desigen Reddy, Srijaya K. Reddy, Megan Rodgers McCormick, Remigio (Remi) Roque, David Rosen, Elizabeth Rossmann Beel, Leelach Rothschild, Lina Sarmiento, Anna Shadrina, Robert Shaw, Michelle Sheth, Allan F Simpao, Neeta Singh, Timothy E Smith, Codruta Soneru, Claire Soria, Peter Szmuk, Brad M. Taicher, Gee Mei Tan, Howard Teng, Thejovathi Edala, Nathaniel Tighe, Simon Tom, Alexander Trujillo, Susan R Vishneski, Juan Pablo Vivas, Adam Von Samek, Britta S von Ungern‐Sternberg, Simon Whyte, Robert T. Wilder
List of Pediatric Anesthesia COVID‐19 Collaborative to be included in PubMed collaborator and institutions listing is provided in two supplementary files.
Funding information
Boston Children's Hospital Internal Departmental Funding.
the Pediatric Anesthesia COVID‐19 Collaborative group members are shown in Appendix 1.
Contributor Information
Petra M. Meier, Email: petra.meier-haran@childrens.harvard.edu.
the Pediatric Anesthesia COVID‐19 Collaborative:
Sindu Balakrishnan, Vipin Bansal, Angela Becerra Torres, Amy Beethe, Hubert A Benzon, Angelina Bhandari, Ashley Bocanegra, Dylan Bould, Melissa Brooks Peterson, Alyssa Brzenski, Veronica Busso, James G Cain, Myles Cassidy, Eric C Cheon, Surendrasingh Chhabada, Lynnie R Correll, Nicholas M Dalesio, Andrew Davidson, Courtney Derderian, Vipul Dhumak, Nicola Disma, Ajay D'Mello, Piedad Echeverry, Pavithra R Ellison, Thomas Erb, Angelica Fajardo, Ricardo J Falcon, Brian Frugoni, Javier García, Olga Lucía Giraldo, Chris D Glover, Jessica Goeller, Susan M Goobie, Ingrid Gooch, Lina Maria Granados, Anastasia Grivoyannis, Velu Guruswamy, Emily Hesselink, Jill Hobbs, Agnes Hunyady, Ranu Jain, Lydia Jorge‐Reynolds, Meredith A Kato, Michael R King, Jamie Kitzman, Jeffrey Koh, Andy Lester, Amanda Lorinc, Constanza Lozano, Katherine Manupipatpong, Clyde Matava, Duncan McLuckie, Kanwal Merchant, Heather Mitzel Levy, Bridget L Muldowney, Julian Andres Navarro, Jonathon Nelson, Amish Patel, Roshan Patel, Niroop Ravula, Desigen Reddy, Srijaya K Reddy, Megan Rodgers McCormick, Remigio (Remi) Roque, David Rosen, Elizabeth Rossmann Beel, Leelach Rothschild, Lina Sarmiento, Anna Shadrina, Robert Shaw, Michelle Sheth, Allan F Simpao, Neeta Singh, Timothy E Smith, Claire Soria, Peter Szmuk, Brad M. Taicher, Gee Mei Tan, Howard Teng, Thejovathi Edala, Nathaniel Tighe, Simon Tom, Alexander Trujillo, Susan R Vishneski, Juan Pablo Vivas, Adam Von Samek, Britta S von Ungern‐Sternberg, Simon Whyte, and Robert T. Wilder
DATA AVAILABILITY STATEMENT
The data that support the findings of this study may be available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time [published correction appears in Lancet Infect Dis. 2020 Sep;20(9):e215]. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Children and COVID‐19: State Data Report. A joint report from the American Academy of Pediatrics and the Children’s Hospital Association. American Academy of Pediatrics and the Children’s Hospital Association, vol. Version:. 2020:11/26/20 https://services.aap.org/en/pages/2019‐novel‐coronavirus‐covid‐19‐infections/children‐and‐covid‐19‐state‐level‐data‐report/.
- 4. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 5. Lin EE, Blumberg TJ, Adler AC, et al. Incidence of COVID‐19 in pediatric surgical patients among 3 US Children’s Hospitals. JAMA Surg. 2020;155(8):775‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee‐Archer P, von Ungern‐Sternberg BS. Pediatric anesthetic implications of COVID‐19‐A review of current literature. Paediatr Anaesth. 2020;30(6):136‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hospitals and Health Systems Face Unprecedented Financial Pressure Due to COVID‐19. American Hospital Association. Accessed Nov 30. 2020;May 5:2020. https://www.aha.org/guidesreports/2020‐05‐05‐hospitals‐and‐health‐systems‐face‐unprecedented‐financial‐pressures‐due. https://www.aha.org/guidesreports/2020‐05‐05‐hospitals‐and‐health‐systems‐face‐unprecedented‐financial‐pressures‐due.
- 8. Matava CT, Kovatsis PG, Lee JK, et al. Pediatric airway management in COVID‐19 patients: consensus guidelines from the society for pediatric anesthesia's pediatric difficult intubation collaborative and the Canadian pediatric anesthesia society. Anesth Analg. 2020;131(1):61‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soneru CN, Nunez K, Petersen TR, Lock R. Anesthetic concerns for pediatric patients in the era of COVID‐19. Paediatr Anaesth. 2020;30(7):737‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okonkwo INC, Howie A, Parry C, et al. The safety of paediatric surgery between COVID‐19 surges: an observational study. Anaesthesia. 2020;75(12):1605‐1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shih G, Deer JD, Lau J, Loveland Baptist L, Lim DJ, Lockman JL. The impact of the COVID‐19 pandemic on the education and wellness of U.S. Pediatric Anesthesiology Fellows [published online ahead of print, 2020 Dec 20]. Paediatr Anaesth. 2020;10. [DOI] [PubMed] [Google Scholar]
- 12. Long T, Soriano S. COVID‐19 a ‘game changer’ for pediatric anesthesia. ASA Monitor. 2020;84:1‐3.https://pubs.asahq.org/monitor/article/84/7/1/108590/COVID‐19‐a‐Game‐Changer‐for‐Pediatric‐Anesthesia . [Google Scholar]
- 13. Berlin G, Bueno D, Gibler K, Schulz J. Cutting through the COVID‐19 surgical backlog. McKinsey & Company. Accessed Nov 30. 2020. October 2:2020. https://www.mckinsey.com/industries/healthcare‐systems‐and‐services/our‐insights/cutting‐through‐the‐covid‐19‐surgical‐backlog#.
- 14. American Society of Anesthesiologists and the Anesthesia Patients Safety Foundation Statement on Perioperative Testing for the COVID‐19 Virus. Anesthesia Patient Safety Foundation. Accessed December 20, 2020. December 8, 2020. https://www.apsf.org/news‐updates/asa‐and‐apsf‐joint‐statement‐on‐perioperative‐testing‐for‐the‐covid‐19‐virus/
- 15. Stock AD, Bader ER, Cezayirli P, et al. COVID‐19 infection among healthcare workers: serological findings supporting routine testing. Front Med (Lausanne). 2020;7:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Society of Anesthesiologists . Purchase and Wearing of Personal Protective Equipment by Anesthesiologists and other Anesthesia Professionals. Accessed December 20, 2020. April 1, 2020. https://www.asahq.org/about‐asa/newsroom/news‐releases/2020/04/purchase‐and‐wearing‐of‐personal‐protective‐equipment‐by‐anesthesiologists‐and‐other‐anesthesia‐professionals.
- 17. Zucco L, Levy N, Ketchandji K, et al. An update on the perioperative considerations for COVID‐19 severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). Anesth Pat Safe Found. 2020;35(2):33‐39. Accessed December 20, 2020 https://www.apsf.org/article/an‐update‐on‐the‐perioperative‐considerations‐for‐covid‐19‐severe‐acute‐respiratory‐syndrome‐coronavirus‐2‐sars‐cov‐2/. [Google Scholar]
- 18. Ranney ML, Griffeth V, Jha AK. Critical supply shortages ‐ The need for ventilators and personal protective equipment during the covid‐19 pandemic. N Engl J Med. 2020;382(18):e41. [DOI] [PubMed] [Google Scholar]
- 19. Cook TM, Lennane S. Occupational COVID‐19 risk for anaesthesia and intensive care staff ‐ low‐risk specialties in a high‐risk setting. Anaesthesia. 2021;76(3):295‐300. [DOI] [PubMed] [Google Scholar]
- 20. Begley JL, Lavery KE, Nickson CP, Brewster DJ. The aerosol box for intubation in coronavirus disease 2019 patients: an in‐situ simulation crossover study. Anaesthesia. 2020;75(8):1014‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simpson JP, Wong DN, Verco L, et al. Measurement of airborne particle exposure during simulated tracheal intubation using various proposed aerosol containment devices during the COVID‐19 pandemic. Anaesthesia. 2020;75(12):1587‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaba D Simulation is a critical tool for advancing patient safety—available to everyone regardless of location or resources. Anesth Pat Safe Found Newsl. 2019;33(3):96‐97. Accessed December 20, 2020. https://nam02.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww.apsf.org%2Farticle%2Fsimulation‐is‐a‐critical‐tool‐for‐advancing‐patient‐safety‐available‐to‐everyone‐regardless‐of‐location‐or‐resources%2F&data=04%7C01%7Caferna27%40jhmi.edu%7C65b1f3f7a9024012877f08d8a5217d14%7C9fa4f438b1e6473b803f86f8aedf0dec%7C0%7C0%7C637440910537345233%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMDAiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C1000&sdata=BjaZSfLSpIII0kLCBlwcwWZg5wkSLh2pK5fXSwxMigg%3D&reserved=0. [Google Scholar]
- 23. Miller TR, Radcliff TA. Economic shocks from the novel COVID‐19 pandemic for anesthesiologists and their practices. Anesth Analg. 2020;131(1):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cutler D. How will COVID‐19 affect the health care economy? JAMA. 2020;323(22):2237‐2238. [DOI] [PubMed] [Google Scholar]
- 25. Krukowski RA, Jagsi R, Cardel MI. Academic productivity differences by gender and child age in science, technology, engineering, mathematics, and medicine faculty during the COVID‐19 pandemic. J Womens Health (Larchmt). 2020;10:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study may be available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


