Abstract
Rationale
Coronavirus disease 2019 (COVID‐19) is associated with many clinical manifestations including respiratory failure and cardiovascular compromise.
Objectives
We examine outcomes in critically ill individuals with COVID‐19 who develop atrial tachyarrhythmias.
Methods
We collected data from electrocardiograms and the electronic medical record of COVID‐19 positive (COVID+) and negative (COVID−) individuals admitted to our medical intensive care unit between February 29 and June 28, 2020. We compared clinical and demographic characteristics, new onset atrial tachyarrhythmia, hemodynamic compromise following atrial tachyarrhythmia, and in‐hospital mortality in COVID+ versus COVID−. Hemodynamic compromise was defined as having a new or increased vasopressor requirement or the need for direct current cardioversion for hemodynamic instability within 1 hour of atrial tachyarrhythmia onset.
Results
Of 300 individuals included, 200 were COVID+ and 100 were COVID−. Mean age was 60 ± 16 years, 180 (60%) were males, and 170 (57%) were African American. New onset atrial tachyarrhythmia occurred in 16% of COVID+ and 19% of COVID− individuals (P = .51). When compared to COVID− participants without atrial tachyarrhythmia, COVID+ individuals with new onset atrial tachyarrhythmia had higher mortality after multivariable adjustment (OR 5.0, 95% CI 1.9–13.5). New onset atrial tachyarrhythmia was followed by hemodynamic compromise in 18 COVID+ but no COVID− participants (P = .0001). COVID+ individuals with hemodynamic compromise after atrial tachyarrhythmia required increased ventilatory support at the time of atrial tachyarrhythmia onset.
Conclusions
Atrial tachyarrhythmia is associated with increased mortality in critically ill individuals with COVID‐19, especially those mechanically ventilated. Recognition of this could assist with clinical care for individuals with COVID‐19.
Keywords: atrial arrhythmias, atrial fibrillation, atrial flutter, atrial tachycardia, COVID‐19
Abbreviations
- ARDS
acute respiratory distress syndrome
- COVID−
coronavirus disease 2019 negative
- COVID+
coronavirus disease 2019 positive
- COVID‐19
coronavirus disease 2019
- ECG
electrocardiogram
- hs troponin
high‐sensitivity troponin
- NE Eq
norepinephrine equivalents
- PEEP
positive end expiratory pressure
- UAB
University of Alabama at Birmingham.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), the disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2, can present with a wide range of clinical manifestations but most commonly with severe respiratory failure. 1 A significant incidence of cardiovascular morbidity and mortality related to COVID‐19 infection is now also recognized. 2 , 3 , 4 , 5 We previously published an analysis showing a high incidence of atrial tachyarrhythmia in critically ill COVID‐19 individuals. 6 This is similar to the incidence published in prior cohorts of critically ill patients without COVID‐19. 7 The development of new onset atrial tachyarrhythmia has been associated with increased incidence of heart failure, stroke, and death in critically ill patients. 8 , 9 , 10 , 11 In this study, we report an analysis of consecutive critically ill participants that required intensive care unit (ICU) admission at a single center with a high degree of suspicion for COVID‐19 infection. We describe the incidence of new‐onset atrial tachyarrhythmia and the association of atrial tachyarrhythmia with short‐term hemodynamic sequelae.
2. METHODS
2.1. Study population
We initially included 215 consecutive individuals age >18 years who were admitted to the medical ICU at the University of Alabama at Birmingham (UAB) Hospital that tested positive for COVID‐19 (COVID+) between February 29 and June 28, 2020. In addition, we included 110 patients who were admitted with a suspicion of COVID‐19 infection between March 13 and April 25, 2020, but who subsequently tested negative (COVID−) by nasopharyngeal swab polymerase chain reaction assay. After excluding 15 patients who were COVID+ and 10 individuals who were COVID− who had a diagnosis of permanent atrial fibrillation on admission, 200 COVID+ and 100 COVID− patients were analyzed. Participants in each group were subsequently subclassified by the presence or absence of new onset atrial tachyarrhythmia (Figure 1). The project was approved by the UAB Institutional Review Board with waiver of informed consent.
FIGURE 1.
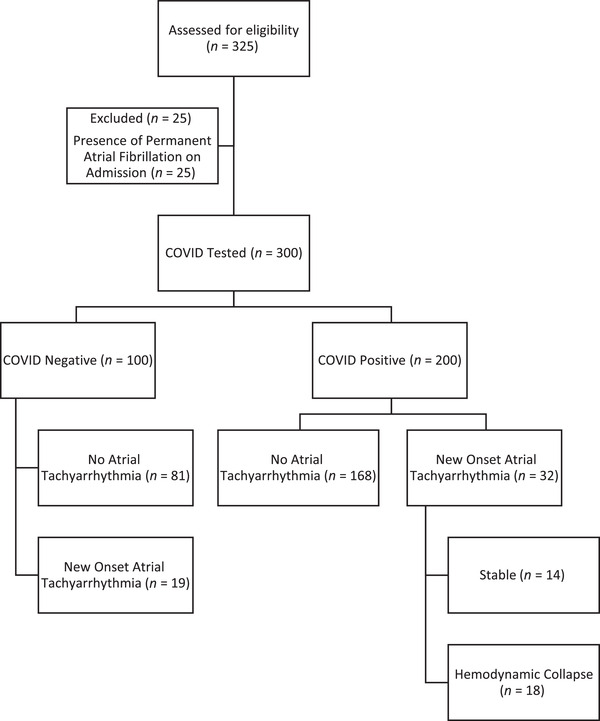
Flowchart describing inclusion process for participant
2.2. Clinical data
Participant demographic information, past medical history, comorbidities, inflammatory markers, high‐sensitivity (hs) troponin levels, outpatient medications, and inpatient therapies were collected from the electronic medical record. In addition, data on the need for mechanical ventilation, duration of mechanical ventilation, ICU and hospital lengths of stay (LOS), and in‐hospital mortality was obtained. To standardize vasopressor dosing across participants receiving different agents, the cumulative dose of norepinephrine equivalents (NE Eq) was calculated as has been described previously 12 , 13 with a conversion factor for angiotensin II of 1 ng per 0.1 μg of norepinephrine based upon the ATHOS‐3 trial (Table S1). 13 All patients admitted to the ICU were monitored with continuous, centrally monitored telemetry. Any change in clinical rhythm was confirmed with 12‐lead electrocardiograms (ECGs). All 12‐lead ECGs recorded were reviewed by a board‐certified cardiologist to determine the development of atrial arrhythmias. Participants with ECG documentation of a new‐onset atrial fibrillation, atrial flutter, or atrial tachycardia were labeled as having an atrial tachyarrhythmia.
TABLE 1.
Demographic and clinical characteristics of critically ill participants overall and by COVID‐19 status, February–June 2020
| Total (n = 300) | COVID+ (n = 200) | COVID− (n = 100) | P value a | |
|---|---|---|---|---|
| Age (years) | 60 ± 16 | 60 ± 16 | 60 ± 16 | .69 |
| BMI (kg/m2) | 31 ± 12 | 32 ± 9 | 30 ± 17 | .25 |
| Male | 180 (60%) | 120 (60%) | 60 (60%) | 1.0 |
| Caucasian | 109 (36%) | 61 (31 %) | 48 (48%) | .005 |
| African American | 170 (57%) | 121 (61%) | 49 (49%) | |
| Other | 21 (7%) | 18 (9%) | 3 (3%) | |
| Clinical characteristics | ||||
| Hypertension | 205 (68%) | 143 (72%) | 62 (62%) | .10 |
| Systolic HF | 44 (15%) | 17 (9%) | 27 (27%) | .0001 |
| Diastolic HF | 68 (23%) | 34 (17%) | 34 (34%) | .001 |
| Coronary artery disease | 59 (20%) | 33 (17%) | 26 (26%) | .05 |
| Atrial fibrillation | 23 (7%) | 9 (5%) | 14 (14%) | .004 |
| Diabetes | 127 (42%) | 93 (47%) | 34 (34%) | .04 |
| Chronic kidney disease | 51 (17%) | 28 (14%) | 23 (23%) | .05 |
| Stroke | 36 (12%) | 19 (10%) | 17 (17%) | .06 |
| COPD | 54 (18%) | 24 (12%) | 30 (30%) | .0001 |
| Cirrhosis | 10 (3%) | 1 (1%) | 9 (9%) | .0001 |
| Obstructive sleep apnea | 35 (12%) | 23 (12%) | 12 (12%) | .90 |
| Tobacco abuse | 102 (34%) | 52 (26%) | 50 (50%) | .0001 |
| Outpatient medications | ||||
| ACE‐I or ARB | 85 (28%) | 56 (28%) | 29 (29%) | .88 |
| Beta‐blocker | 103 (34%) | 56 (28%) | 47 (47%) | .001 |
| Aspirin | 86 (28%) | 53 (27%) | 33 (33%) | .25 |
| P2Y12 inhibitor | 25 (8%) | 15 (8%) | 10 (10%) | .47 |
| Peak inpatient laboratory values | ||||
| D‐dimer (ng/mL) | 3100 ± 4232 | 3542 ± 4664 | 1861 ± 2290 | .02 |
| CRP (mg/L) | 176 ± 116 | 200 ± 113 | 93 ± 86 | .0001 |
| BNP (pg/mL) | 474 ± 690 | 331 ± 566 | 732 ± 812 | .0001 |
| hs troponin (ng/L) | 968 ± 4557 | 549 ± 1599 | 1906 ± 7795 | .03 |
| Inpatient therapies | ||||
| Vasopressor use | 180 (60%) | 142 (71%) | 38 (38%) | .0001 |
| Mechanical ventilation | 190 (63%) | 150 (75%) | 40 (40%) | .0001 |
| Outcomes | ||||
| New ATA | 51 (17%) | 32 (16%) | 19 (19%) | .51 |
| Death | 86 (29%) | 66 (33%) | 20 (20%) | .02 |
Values are mean ± SD or n (%).
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ATA, atrial tachyarrhythmia; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; COVID, coronavirus disease; CRP, c‐reactive protein; diastolic HF, diastolic heart failure; hs troponin, high‐sensitivity troponin; systolic HF, systolic heart failure.
a P value comparing COVID+ versus COVID−.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.3. Outcomes
We collected data on in‐hospital mortality as well the development of hemodynamic compromise following atrial tachyarrhythmia. To determine hemodynamic compromise in those with new‐onset atrial tachyarrhythmia, the maximum NE Eq doses of vasopressor recorded the hour before the onset of atrial tachyarrhythmia were compared with the maximum NE Eq dose of vasopressors during the hour after the onset of these arrhythmias. Participants were classified as having hemodynamic compromise following atrial tachyarrhythmia if their NE Eq vasopressor dose requirement increased or if direct current cardioversion was performed within 1 hour of acute arrhythmia onset.
2.4. Statistical analyses
Continuous variables are presented as mean ± standard deviation and compared using independent samples t‐tests. Categorical variables were expressed as frequencies and compared using chi‐square tests. To examine mortality, we divided our study population into four groups: (1) COVID+ with atrial tachyarrhythmia, (2) COVID+ without atrial tachyarrhythmia, (3) COVID− with atrial tachyarrhythmia, and (4) COVID− without atrial tachyarrhythmia. Individual logistic regression models were constructed using the COVID− without atrial tachyarrhythmia group as the referent. Models were adjusted for age, sex, race, body mass index, and clinical characteristics that varied by COVID status (systolic heart failure, diastolic heart failure, atrial fibrillation, chronic kidney disease, diabetes, chronic obstructive pulmonary disease, cirrhosis, and tobacco use). All tests were two‐tailed, and a P value < .05 (set a priori) was considered statistically significant. All statistical analyses and graphics creations were performed using SPSS Statistics version 26 software (IBM Corp., Armonk, NY, USA) and GraphPad Prism v. 7.0 (GraphPad Software, San Diego, CA, USA).
3. RESULTS
The baseline characteristics of those with and without COVID‐19 are shown in Table 1. The cohort had many features that put them at high risk for COVID‐19 complications with a mean age of 60 ± 16 years, 180 (60%) were men, 170 (57%) were African American, 109 (36%) were Caucasian, and there were high rates of underlying chronic metabolic, pulmonary, renal, and cardiovascular comorbidities that have been associated with poor outcome in other published COVID‐19 cohorts. 5 , 14 , 15 , 16 Compared to those who were COVID−, COVID+ participants were more likely to have a history of diabetes (47% vs. 34%, P = .04), but less likely to have a systolic heart failure (9% vs. 27%, P = .001), diastolic dysfunction (17% vs. 34%, P = .001), paroxysmal atrial fibrillation (5% vs. 14%, P = .004), chronic kidney disease (14% vs. 23%, P = .05), chronic obstructive pulmonary disease (12% vs. 30%, P = .0001), tobacco abuse (26% vs. 50%, P = .0001), and cirrhosis (1% vs. 9%, P = .001). COVID+ participants were less likely to be prescribed beta blockers in the outpatient setting (28% vs. 47%, P = .001). COVID+ participants also had higher D‐dimer (3542 ± 4664 ng/mL vs. 1861 ± 2290 ng/mL, P = .02) and CRP levels (200 ± 113 mg/L vs. 93 ± 86 mg/L, P = .0001) but lower brain natriuretic peptide (331 ± 566 pg/mL vs. 732 ± 812 pg/mL, P = .0001) and hs troponin (549 ± 1599 ng/L vs. 1906 ± 7795 ng/L, P = .03) levels. Individuals with COVID‐19 were more likely to require vasopressor support (71% vs. 38%, P = .0001) and mechanical ventilation (75% vs. 40%, P = .0001) than those who were COVID−. COVID+ therapies included azithromycin in 150 participants, hydroxychloroquine in 7 participants, and remdesivir in 78 participants. An atrial tachyarrhythmia was recorded by 12‐lead ECG in 32 COVID+ participants (16%) and 19 COVID− participants (19%). Atrial tachyarrhythmias included atrial fibrillation in 34 participants, atrial flutter in 14 participants, and atrial tachycardia in 3 participants. There were no statistically significant differences in demographic or baseline comorbid conditions for participants with atrial tachyarrhythmias compared to those without.
In‐hospital mortality by COVID status in those with and without atrial tachyarrhythmia is shown in Table 2. Individuals who were COVID+ with new onset atrial tachyarrhythmia had the highest in‐hospital mortality (50%), followed by those who were COVID+ without atrial tachyarrhythmia (30%), COVID− individuals with new onset atrial tachyarrhythmia (26%), and COVID− patients without atrial tachyarrhythmia (19%) (P = .01). When compared to those who were COVID− without new onset atrial tachyarrhythmia, individuals who were COVID+ with new onset atrial tachyarrhythmia had higher in‐hospital mortality in both unadjusted (OR 4.4, 95% CI 1.8–10.7) and multivariable adjusted (OR 5.0, 95% CI 1.9–13.5) models. Individuals who were COVID− with new onset atrial tachyarrhythmia also had increased in‐hospital mortality when compared to those who were COVID− without new onset atrial tachyarrhythmia after multivariable adjustment (OR 2.3, 95% CI 1.1–5.0) although the magnitude of this association was less than for those who were COVID+ with new onset atrial tachyarrhythmia.
TABLE 2.
In‐hospital mortality by COVID status in those with and without new onset atrial tachyarrhythmia, February–June 2020
| Unadjusted | Adjusted a | ||||||
|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||
| Odds ratio | Lower | Upper | Odds ratio | Lower | Upper | ||
| COVID+ ATA | 16 (50%) | 4.4 | 1.8 | 10.7 | 5.0 | 1.9 | 13.5 |
| COVID+ NO ATA | 50 (30%) | 1.9 | 1.0 | 3.6 | 1.6 | 0.4 | 5.7 |
| COVID− ATA | 5 (26%) | 1.5 | 0.5 | 5.0 | 2.3 | 1.1 | 5.0 |
| COVID− NO ATA | 15 (19%) | Referent | Referent | ||||
Abbreviations: ATA, atrial tachyarrhythmia; CI, confidence interval; COVID, coronavirus disease.
aAdjusted for age, gender, race, body mass index, systolic heart failure, diastolic heart failure, atrial fibrillation, chronic kidney disease, diabetes, chronic obstructive pulmonary disease, cirrhosis, and tobacco use.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The demographic and clinical characteristics and inpatient therapies for individuals with atrial tachyarrhythmias are shown in Table 3. Those who were COVID+ and developed an atrial tachyarrhythmia were more likely to require vasopressors (91% vs. 47%, P = .001), had a longer duration on vasopressors (9 ± 6 days vs. 2 ± 2 days, P = .0001), more likely to require mechanical ventilation (94% vs. 42%, P = .0001), had a longer duration of mechanical ventilation (18 ± 11 days vs. 4 ± 9 days, P = .0001), had longer ICU LOS (23 ± 8 days vs. 12 ± 11 days, P = .0001), and had longer hospital LOS (25 ± 6 days vs. 17 ± 9 days, P = .0001) compared to those who were COVID− and developed an atrial tachyarrhythmia.
TABLE 3.
Demographics and clinical characteristics of critically ill participants with new onset atrial tachyarrhythmias by COVID‐19 status, February–June 2020
| COVID+ ATA (n = 32) | COVID− ATA (n = 19) | P value | |
|---|---|---|---|
| Age (years) | 65 ± 13 | 64 ± 15 | .88 |
| BMI (kg/m2) | 29 ± 8 | 27 ± 7 | .37 |
| Male | 21 (66%) | 12 (63%) | .82 |
| White | 15 (47%) | 11 (58%) | .45 |
| Inpatient therapies | |||
| Vasopressor use | 29 (91%) | 9 (47%) | .00 |
| Vasopressor days | 9 ± 6 | 2 ± 2 | .0001 |
| Max NE equation (μg/kg/min) | 0.45 ± 0.51 | 0.28 ± 0.43 | .22 |
| Mechanical ventilation | 30 (94%) | 8 (42%) | .0001 |
| Days on MV | 18 ± 11 | 4 ± 9 | .0001 |
| Outcomes | |||
| ICU LOS (days) | 23 ± 8 | 12 ± 11 | .0001 |
| Hospital LOS (days) | 25 ± 6 | 17 ± 9 | .0001 |
| Death | 16 (50%) | 5 (26%) | .10 |
Values are mean ± SD or n (%).
Abbreviations: ATA, atrial tachyarrhythmia; BMI, body mass index; COVID, coronarvirus disease; hospital LOS, hospital length of stay; ICU LOS, intensive care unit length of stay; max, maximum; MV, mechanical ventilation; NE Eq, norepinephrine equivalents.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Hemodynamic compromise occurred in 18 participants in the COVID+ group and none in the COVID− group (P = .0001). Among the 18 COVID+ individuals who experienced hemodynamic compromise, 17 experienced an increasing NE Eq requirement and 1 required immediate direct current cardioversion for hemodynamic instability at atrial tachyarrhythmia onset. In the 17 participants that had an increase in vasopressor requirement, the average change in NE Eq was 0.18 μg/kg/min. A graphical representation of NE Eq dosage changes can be found in Figure 2. Atrial tachyarrhythmia was treated with amiodarone in 29 (57%) participants, beta blockers in 38 (75%), calcium channel blocker in 5 (10%), and anticoagulation was felt to be safe in 31 (61%) participants.
FIGURE 2.
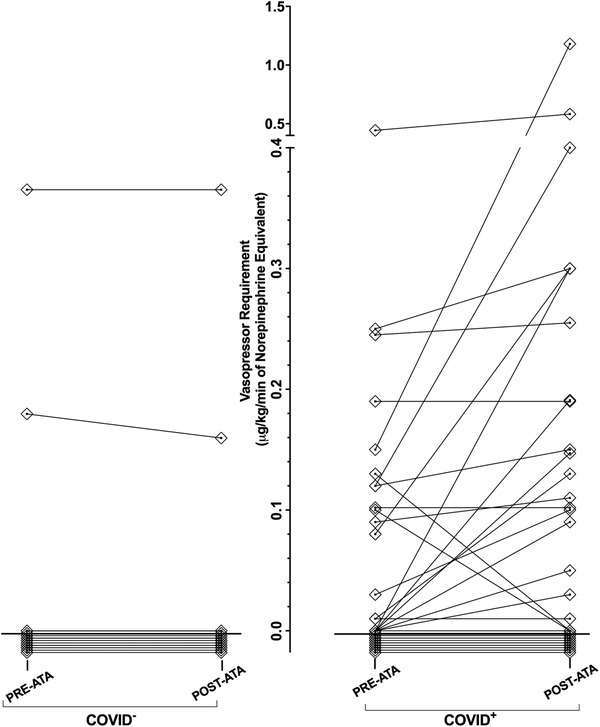
Norepinephrine equivalent doses of vasopressors in critically ill COVID+ and COVID− Individuals in the 1 hour before and the 1 hour after atrial tachyarrhythmia onset, February–June 2020. In the COVID− cohort, 17 patients had no increase or change in vasopressor use, 1 individual having no change in vasopressor dose, and 1 individual had a decrease in vasopressor dose following ATA onset. In the COVID+ cohort, 11 individuals were not on a vasopressor and did not require the addition of a vasopressor, 2 individuals had no change in vasopressor dose, 17 individuals saw an increasing vasopressor requirement, 1 individual was taken off vasopressors after ATA onset, and 1 individual was taken off vasopressors after DCCV following ATA onset
Abbreviations: ATA, atrial tachyarrhythmia; COVID, coronavirus disease; DCCV, direct current cardioversion
Characteristics of participants with new onset atrial tachyarrhythmia by hemodynamic status are shown in Table 4. When compared to the 14 COVID+ hemodynamically stable participants following atrial tachyarrhythmia onset, the 18 COVID+ participants who developed hemodynamic compromise after atrial tachyarrhythmia onset had similar comorbid conditions and baseline echocardiographic assessment with a lower mean arterial pressure (74 ± 16 vs. 89 ± 10, P = .004), higher serum potassium (4.5 ± 0.4 vs. 4.2 ± 0.5, P = .04), greater vasopressor use (83% vs. 21%, P = .0001), greater need for mechanical ventilation (100% vs. 57%, P = .002), higher positive end expiratory pressure (PEEP) requirements (10 ± 4 vs. 5 ± 4, P = .005), and increased in‐hospital mortality (67% vs. 29%, P = .03). In fact, of the 16 individuals with COVID‐19 and a new onset atrial tachyarrhythmia who subsequently died, 12 (75%) had hemodynamic compromise immediately after developing the atrial tachyarrhythmia.
TABLE 4.
Demographic and clinical characteristics of critically ill participants who were hemodynamically stable or unstable following atrial tachyarrhythmia onset by COVID‐19 status, February–June 2020
| COVID+ (n = 32) | COVID− (n = 19) | ||||
|---|---|---|---|---|---|
| HC (n = 18) | Stable (n = 14) | P value a | Stable (n = 19) | P value b | |
| Age (years) | 64 ± 15 | 62 ± 12 | .63 | 64 ± 15 | .92 |
| BMI (kg/m2) | 30 ± 8 | 27 ± 6 | .27 | 27 ± 7 | .24 |
| Male | 11 (61%) | 10 (71%) | .54 | 12 (63%) | .90 |
| White | 9 (50 %) | 8 (57%) | .69 | 11 (58%) | .63 |
| Past medical history | |||||
| Hypertension | 14 (78%) | 9 (64%) | .40 | 13 (68%) | .19 |
| Systolic HF | 2 (11%) | 3 (21%) | .43 | 5 (26%) | .24 |
| Diastolic HF | 2 (11%) | 4 (29%) | .21 | 9 (47%) | .02 |
| Coronary artery disease | 2 (11%) | 3 (21%) | .43 | 8 (42%) | .03 |
| Atrial fibrillation | 1 (6%) | 3 (21%) | .18 | 5 (26%) | .09 |
| Diabetes | 10 (56%) | 4 (29%) | .13 | 6 (32%) | .14 |
| COPD | 3 (17%) | 2 (14%) | .85 | 7 (37%) | .17 |
| Echocardiography | |||||
| LVEF | 55 ± 14 | 58 ± 10 | .62 | 44 ± 17 | .07 |
| Diastolic dysfunction | 4 (22%) | 3 (30%) | .78 | 11 (92%) | .0001 |
| LVIDd | 4.7 ± 0.8 | 4.9 ± 0.3 | .41 | 5.3 ± 1.4 | .16 |
| RV dysfunction | 5 (28%) | 1 (10%) | .21 | 1 (10%) | .21 |
| TAPSE | 2.0 ± 0.4 | 2.2 ± 0.5 | .38 | 2.0 ± 0.4 | .96 |
| LA dilation | 2 (11%) | 2 (25%) | .65 | 3 (27%) | .54 |
| Mitral regurgitation | 3 (17%) | 4 (29%) | .42 | 5 (26%) | .48 |
| Tricuspid regurgitation | 5 (28%) | 6 (43%) | .37 | 7 (37%) | .56 |
| Aortic regurgitation | 2 (11%) | 0 | .20 | 4 (21%) | .41 |
| Pericardial effusion | 4 (22%) | 3 (21%) | .96 | 3 (16%) | .62 |
| Vital signs and laboratory values at ATA onset | |||||
| Heart rate change | 50 ± 22 | 35 ± 24 | .09 | 39 ± 25 | .18 |
| MAP | 74 ± 16 | 89 ± 10 | .004 | 82 ± 15 | .12 |
| Sodium | 140 ± 6 | 137 ± 5 | 0.34 | 138 ± 8 | .49 |
| Potassium | 4.5 ± 0.4 | 4.2 ± 0.5 | .04 | 4.1 ± 0.6 | .02 |
| Magnesium | 2.0 ± 0.4 | 2.2 ± 0.4 | .21 | 2.0 ± 0.5 | .99 |
| Creatinine | 2.2 ± 1.4 | 1.8 ± 0.8 | .46 | 2.2 ± 2.4 | .90 |
| Inpatient therapies at ATA onset | |||||
| Mechanical ventilation | 18 (100%) | 8 (57%) | .002 | 3 (16%) | .0001 |
| Prone positioning | 2 (11%) | 0 | .20 | 0 | .14 |
| Vasopressors use | 15 (83%) | 3 (21%) | .0001 | 2 (11%) | .0001 |
| CRRT | 9 (50%) | 4 (29%) | .22 | 4 (21%) | .07 |
| Ventilation parameters at ATA onset c | |||||
| PEEP | 10 ± 4 | 5 ± 4 | .005 | 1 ± 3 | .0001 |
| Plateau pressure | 25 ± 7 | 23 ± 5 | .58 | 27 ± 4 | .75 |
| FiO2 (%) | 57 ± 17 | 46 ± 15 | .06 | 29 ± 6 | .0001 |
| Outcomes | |||||
| Death | 12 (67%) | 4 (29%) | .03 | 5 (26%) | .01 |
Values are mean ± SD or n (%).
Abbreviations: ATA, atrial tachyarrhythmia; BMI, body mass index; COPD, chronic obstructive pulmonary disease; COVID, coronavirus disease; CRRT, continuous renal replacement therapy; FiO2, fraction of inspired oxygen; LA dilation, left atrial dilation; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diameter end diastole; MAP, mean arterial pressure; PEEP, positive end expiratory pressure; TAPSE, tricuspid annular plane systolic excursion.
a P value comparing COVID+ hemodynamic collapse group versus COVID+ stable group.
P value comparing COVID+ hemodynamic collapse group versus COVID− stable group.
cIndividuals who were on room air at the time of analysis were given a PEEP value of 0 and an FiO2 value of 0.21 + Oxygen in L/min × 0.03. No plateau pressure was recorded for patients not ventilated.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
When compared to the 19 COVID− participants who remained hemodynamically stable following atrial tachyarrhythmia onset, the 18 COVID+ participants who developed hemodynamic compromise after atrial tachyarrhythmia onset had a decreased prevalence of past diastolic dysfunction (11% vs. 47%, P = .02) and coronary artery disease (11% vs. 42%, P = .03) but a higher serum potassium (4.5 ± 0.4 vs. 4.1 ± 0.6, P = .02), greater need for vasopressor use (83% vs. 11%, P = .0001), greater need for mechanical ventilation (100% vs. 16%, P = .0001), higher PEEP (10 ± 4 mmHg vs. 1 ± 3 mmHg, P = .0001), higher fraction of inspired oxygen requirements (57 ± 17 vs. 29 ± 6, P = .0001), and increased in‐hospital mortality (67% vs. 29%, P = .01).
4. DISCUSSION
In this study, critically ill COVID+ and COVID− individuals with new onset atrial tachyarrhythmia had increased in‐hospital mortality when compared to those without atrial tachyarrhythmia, although the magnitude of this association was greater for those who were COVID+. In addition, we observed a temporal relationship between new onset atrial tachyarrhythmia and hemodynamic compromise in individuals who were COVID+ which might explain their increased in‐hospital mortality. In fact, of the 16 individuals with COVID‐19 and a new onset atrial tachyarrhythmia who subsequently died, 12 (75%) had hemodynamic compromise immediately after developing the atrial tachyarrhythmia.
Atrial tachyarrhythmia in critically ill individuals is thought to be driven by both individual factors such as myocardial dysfunction due to infection, drugs, and cytokine levels 19 as well as by critical care interventions such as vasopressor use and mechanical ventilation. 20 , 21 , 22 , 23 The occurrence of atrial tachyarrhythmia during critical illness has been associated with poor outcomes, including increased hospital mortality, 9 increased duration of ICU admission, and 1‐year adjusted survival. 7 However, to our knowledge, the consequences of atrial tachyarrhythmia in COVID‐19 related critical illness have not been previously reported.
We found that the short‐term effect of atrial tachyarrhythmia on COVID+ participants was distinct from that seen in those who were COVID−, with a marked temporal correlation between onset of atrial tachyarrhythmia and hemodynamic compromise seen uniquely among a group of COVID+ individuals who were mechanically ventilated and requiring significant levels of ventilator support. COVID+ participants who developed atrial tachyarrhythmia with concurrent respiratory failure appeared much more vulnerable to hemodynamic compromise just after atrial tachyarrhythmia onset, suggesting an increased hemodynamic sensitivity of mechanically ventilated COVID+ individuals to loss of sinus rhythm relative to COVID− critically ill participants. Despite the known association of severe COVID‐19 infection with cardiovascular comorbid diseases, COVID+ participants actually had a lower burden of chronic cardiac disease and valvular disease, and a higher ejection fraction compared to the COVID− group, arguing that structural heart disease is not the reason for their apparent hemodynamic sensitivity to the loss of sinus rhythm. Rather, our data suggest that the striking relationship of hemodynamic deterioration to new onset atrial tachyarrhythmia in COVID+ individuals may be related to cardiopulmonary interactions in severe acute respiratory distress syndrome (ARDS) and/or to the high degree of ventilator support that they require, including high PEEP support.
Previous studies have shown that increasing PEEP is associated with decreased cardiac output and mean blood pressure. 24 We speculate that the loss of atrial contractility in individuals with COVID‐19 ARDS may further decrease preload and cause hemodynamic decompensation. This is further supported by the high prevalence of mechanical ventilation and subsequent temporal decompensation observed at onset of atrial tachyarrhythmia. Moreover, recent studies have highlighted the importance of right ventricular longitudinal strain in individuals with ARDS as a predictor of mortality highlighting the importance of right heart function and clinical outcomes. 25 , 26
These findings carry several important implications. This study suggests a potential causal relationship between atrial tachyarrhythmia onset and hemodynamic instability in COVID+ individuals. Importantly, the high mortality associated with ARDS appears to be driven more strongly by hemodynamic instability and degree of shock than by hypoxemia, 27 therefore a complication so closely associated with marked hemodynamic deterioration may significantly influence outcomes. Indeed, participants with atrial tachyarrhythmia associated hemodynamic compromise did have worsened survival in our study. We hypothesize that vigilance to optimize factors that may increase the risk of atrial tachyarrhythmia, such as electrolyte imbalances and volume overload, may be beneficial not only for heart rhythm, but also for blood pressure stability and downstream outcomes including survival. Although these findings may suggest that less hemodynamically impactful ventilatory strategies, such as a low PEEP strategy, could improve hemodynamic stability in COVID+ individuals or ARDS individuals with atrial tachyarrhythmia, this study does not directly address this question. It is conceivable that increased attention to a rhythm control strategy in COVID‐19 individuals may have greater benefit than that seen in general critical illness, and prospective studies of this question may be justified. As our COVID− comparative cohort did not have a high incidence of ARDS, it is unclear if the observed hemodynamic changes related to atrial tachyarrhythmia are unique to COVID infection and may represent a phenomenon seen in all individuals with severe ARDS. Studies have shown that prone positioning in individuals with ARDS improves ventilation and improves right ventricular ejection fraction, 28 left ventricular preload, 28 , 29 and cardiac output. 30 , 31 Thus, prone positioning may represent another potential approach to attenuate the hemodynamic effects of atrial tachyarrhythmia in COVID‐19, an effect that could conceivably contribute to the survival benefit shown with this agent in ARDS.
While the specific mechanism of myocardial injury in COVID infection remains to be defined, individuals susceptible to atrial arrhythmias and myocardial injury may be more likely to develop severe manifestations of viral infection. 32 It remains to be seen whether early intervention of atrial tachyarrhythmia in these individuals will mitigate the severe clinical course of the disease.
5. LIMITATIONS
There are several significant limitations of our study. First, this is a retrospective study from a single tertiary referral center that likely over represents individuals with severe manifestations of COVID‐19 infection. Secondly, our study has a small sample size with a comparative group that does not perfectly match our COVID+ population. However, our COVID+ population had decreased incidence of comorbid conditions and structural heart abnormalities offering less potential for confounding than our standard intensive care population.
6. CONCLUSIONS
Individuals with COVID‐19 who are admitted to the ICU have a high incidence of new onset atrial tachyarrhythmia that is associated with hemodynamic compromise and death. Special attention should be paid to new onset atrial tachyarrhythmia in COVID+ patients as it appears to be associated with worse outcomes.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose relevant to the content matter being studied or discussed. There are no other grants, contracts, or other forms of financial support to disclose.
AUTHOR CONTRIBUTIONS
Each author contributed via data collection, analysis, and manuscript writing or editing. Each author has reviewed the final manuscript and has approved it.
ACKNOWLEDGMENTS
The work was not supported by industry. This study received internal funding from the Departments of Cardiovascular Disease and Pulmonary, Allergy and Critical Care at the University of Alabama at Birmingham.
Colon CM, Barrios JG, Chiles JW, et al. Atrial arrhythmia‐related outcomes in critically ill COVID‐19 patients. Pacing Clin Electrophysiol. 2021;44:814‐823. 10.1111/pace.14221
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically ill patients in the Seattle region ‐ case series. N Engl J Med. 2020;382:2012‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:819‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colon CM, Barrios JG, Chiles JW, et al. Atrial arrhythmias in COVID‐19 patients. JACC Clin Electrophysiol. 2020;6:1189‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein Klouwenberg PM, Frencken JF, Kuipers S, et al. Incidence, predictors, and outcomes of new‐onset atrial fibrillation in critically ill patients with sepsis. A cohort study. Am J Respir Crit Care Med. 2017;195:205‐211. [DOI] [PubMed] [Google Scholar]
- 8. Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moss TJ, Calland JF, Enfield KB, et al. New‐onset atrial fibrillation in the critically ill. Crit Care Med. 2017;45:790‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaver CM, Chen W, Janz DR, et al. Atrial fibrillation is an independent predictor of mortality in critically ill patients. Crit Care Med. 2015;43:2104‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new‐onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248‐2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Russell JA, Wellman H, Walley KR. Vasopressin versus norepinephrine in septic shock: a propensity score matched efficiency retrospective cohort study in the VASST coordinating center hospital. J Intensive Care. 2018;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377:419‐430. [DOI] [PubMed] [Google Scholar]
- 14. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu Y, Xu D, Fu S, et al. Patients with COVID‐19 in 19 ICUs in Wuhan, China: a cross‐sectional study. Crit Care. 2020;24:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehra MR, Desai SS, Kuy S, TD Henry, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid‐19. N Engl J Med. 2020;382:e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu K, Fang Y‐Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province. Chin Med J (Engl). 2020;133:(9)1025‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bosch NA, Cimini J, Walkey AJ. Atrial fibrillation in the ICU. Chest. 2018;154:1424‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seemann A, Boissier F, Razazi K, et al. New‐onset supraventricular arrhythmia during septic shock: prevalence, risk factors and prognosis. Ann Intensive Care. 2015;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duarte PAD, Leichtweis GE, Andriolo L, et al. Factors associated with the incidence and severity of new‐onset atrial fibrillation in adult critically ill patients. Crit Care Res Pract. 2017;2017:8046240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304‐377. [DOI] [PubMed] [Google Scholar]
- 23. Vieillard‐Baron A, Boyd J. Non‐antiarrhythmic interventions in new onset and paroxysmal sepsis‐related atrial fibrillation. Intensive Care Med. 2018;44:94‐97. [DOI] [PubMed] [Google Scholar]
- 24. Jardin F, Farcot JC, Boisante L, Curien N, Margairaz A, Bourdarias JP. Influence of positive end‐expiratory pressure on left ventricular performance. N Engl J Med. 1981;304:387‐392. [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID‐19. JACC Cardiovasc Imaging. 2020;13:2287‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Argulian E, Sud K, B V, et al. Right ventricular dilation in hospitalized patients with COVID‐19 infection. JACC Cardiovasc Imaging. 2020;13:2459‐2461. 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vieillard‐Baron A, Girou E, Valente E, et al. Predictors of mortality in acute respiratory distress syndrome. Focus on the role of right heart catheterization. Am J Respir Crit Care Med. 2000;161:1597‐1601. [PubMed] [Google Scholar]
- 28. Vieillard‐Baron A, Charron C, Caille V, Belliard G, Page B, Jardin F. Prone positioning unloads the right ventricle in severe ARDS. Chest. 2007;132:1440‐1446. [DOI] [PubMed] [Google Scholar]
- 29. Jozwiak M, Teboul JL, Anguel N, et al. Beneficial hemodynamic effects of prone positioning in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188:1428‐1433. [DOI] [PubMed] [Google Scholar]
- 30. Ruste M, Bitker L, Yonis H, et al. Hemodynamic effects of extended prone position sessions in ARDS. Ann Intensive Care. 2018;8:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hering R, Wrigge H, Vorwerk R, et al. The effects of prone positioning on intraabdominal pressure and cardiovascular and renal function in patients with acute lung injury. Anesth Analg. 2001;92:1226‐1231. [DOI] [PubMed] [Google Scholar]
- 32. Clerkin KJ, Fried JA, Raikhelkar J, et al. Covid‐19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


