Figure 1.
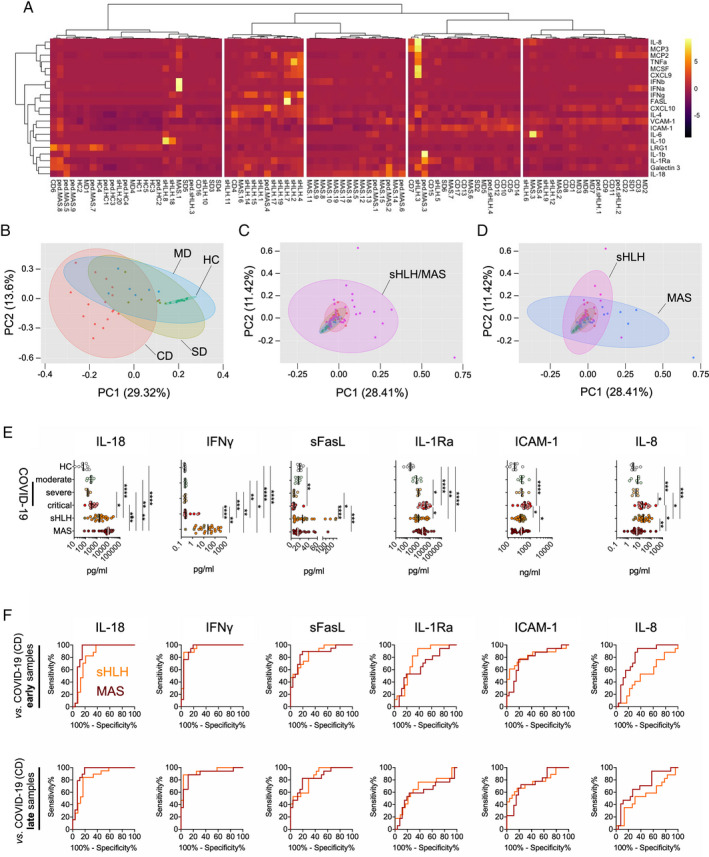
Serum biomarker profiles in patients with COVID‐19 compared to patients with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS). A, Heatmap from unsupervised clustering analysis using correlation distance and ward.D linkage showing biomarker levels in the first serum sample obtained following hospitalization from patients with critical COVID‐19 (CD) (presence of acute respiratory distress syndrome [ARDS] and/or deceased; n = 17), those with severe disease (SD) (requiring oxygen supplementation; n = 6), or those with moderate disease (MD) (ARDS not present and oxygen supplementation not required; n = 7) in relation to measurements of serum biomarker levels in patients with active secondary HLH (adult, n = 18; pediatric, n = 4) and patients with MAS (adult‐onset Still’s disease, n = 17; systemic lupus erythematosus [SLE], n = 2; systemic juvenile idiopathic arthritis, n = 8; juvenile SLE, n = 1) as well as healthy controls (HC) (n = 9). Color coding indicates the row Z score for expression levels in each sample. B–D, Principal components (PC) analyses of the serum samples described in A, analyzing biomarker profiles in serum from patients with COVID‐19 according to disease severity compared to healthy controls (B) and patients with secondary HLH/MAS (C) and from patients with secondary HLH compared to patients with MAS (D). E, Individual biomarkers showing differential expression in patients with COVID‐19 according to disease severity compared to patients with secondary HLH, patients with MAS, and healthy controls. Results are shown as scatterplots, in which symbols represent individual samples, and vertical lines show the median. * = P < 0.05; ** = P < 0.01; *** = P < 0.001; **** = P < 0.0001, by Kruskal‐Wallis test followed by Dunn’s test for multiple comparisons. F, Receiver operating characteristic curve analyses of individual serum biomarkers (corresponding to those in E) for the differentiation of patients with critical COVID‐19 from patients with secondary HLH or MAS. Results are shown according to the time of sample collection from patients with COVID‐19: early = first serum sample obtained following hospitalization; late = later in disease progression. IL‐8 = interleukin‐8; MCP‐3 = monocyte chemoattractant protein 3; TNFA = tumor necrosis factor; MCSF = macrophage colony‐stimulating factor; IFNb = interferon‐β; VCAM‐1 = vascular cell adhesion molecule 1; ICAM‐1 = intercellular adhesion molecule 1; LRG‐1 = leucine‐rich α2‐glycoprotein 1; sFasL = soluble FasL; IL‐1Ra = IL‐1 receptor antagonist.
