Abstract
Maintaining good glycaemic control with the same infusion set for longer than 3 days may improve the quality of life of insulin pump users. The aim of the current study was to assess the efficacy and safety of the novel, extended‐wear infusion set over 7 days of wear in adults with type 1 diabetes. Sixteen participants completed three identical 8‐hour euglycaemic clamp experiments on Days 1, 4 and 7 of infusion set wear. Between the experiments, the participants were discharged home for routine diabetes management while wearing the same extended‐wear infusion set throughout the study. Time to reach the maximum glucose infusion rate (TGIRmax) on Day 7 was reduced by 67% compared with Day 1 (p < .001). The corresponding area under the glucose infusion rate curve (AUCGIR) was comparable for the first 2 h of the clamp (p = .891) but decreased by 28% over time (p < .008). While the extent of insulin absorption decreased with prolonged wear, it was accompanied by an increase in insulin absorption rate. The infusion set survival rate was 100% without leakages, occlusion alarms, severe hypoglycaemia or ketoacidosis. The extended‐wear infusion set proved safe and effective during prolonged wear in real‐life conditions.
Keywords: clinical trial, CSII, insulin pump therapy, pharmacodynamics, pharmacokinetics, type 1 diabetes
1. INTRODUCTION
Modern insulin therapy aims to establish good glycaemic control without causing clinically relevant hypoglycaemia. Currently, continuous subcutaneous insulin infusion (CSII) therapy comes closest to achieving that goal. 1 , 2 , 3 , 4 However, because of physiological and mechanical effects occurring at the infusion site, CSII may be associated with non‐metabolic complications 5 , 6 (i.e. local tissue inflammation, the effect of insulin solution preservatives on the infusion site, infusion set‐related events) that affect insulin delivery leading to fluctuations in insulin absorption and eventually to deterioration of glycaemic control. Because the incidence and frequency of CSII complications increase over time, manufacturers of insulin pumps and infusion sets recommend changing the infusion set and rotating the infusion site every 2–3 days to ensure reliable insulin delivery. 7 , 8 , 9 , 10 , 11 However, in practice, most individuals prefer to use one infusion set and one insertion site longer than recommended to avoid experiencing frequent insertion‐related pain, but also to keep the cost of treatment down.
We investigated the efficacy and safety of the novel, extended‐wear insulin infusion set featuring patented Lantern technology over 7 days of wear. The set includes a soft cannula with anti‐inflammatory coating and additional openings in the form of multiple longitudinal perforations in the cannula shaft. The cannula coating is designed to suppress the foreign body response, while the shaft perforations compensate for fluctuations in insulin delivery because of kinking/crimping, or a partial/full cannula tip occlusion (Figure 1). Owing to the cannula design, the extended‐wear infusion set may reduce the likelihood of non‐metabolic CSII complications, thus enabling prolonged wear, consistent with patients' wishes.
FIGURE 1.
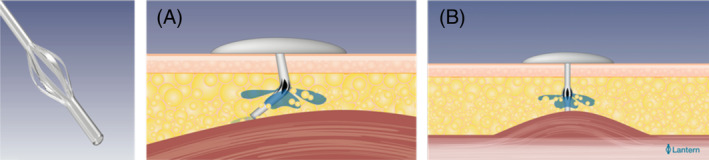
Extended‐wear infusion set cannula with additional openings in the form of multiple longitudinal perforations in the cannula shaft: because of the cannula design, uninterrupted insulin flow is facilitated if A, the cannula is kinked, and B, when the cannula tip is occluded
2. METHODS
This open, single‐centre, single‐arm, controlled pilot study assessed the efficacy and safety of the novel, extended‐wear insulin infusion set featuring Lantern technology in 16 experienced insulin pump users with type 1 diabetes over 7 days of wear. The study was approved by the Medical University of Graz ethics committee (EC number 29–566 ex 16/17) and the Austrian national health authority, and was entered into the German Clinical Trials Register (DRKS00013263). Signed informed consent was obtained prior to any study‐related activities. The participants (five females and 11 males, age 44.2 ± 15.4 years, body mass index 24.5 ± 2.3 kg/m2, diabetes duration 20.0 ± 9.0 years, HbA1c 7.2% ± 0.7% [55.3 ± 7.8 mmol/mol]) underwent a total of four identical 8‐h long euglycaemic clamp experiments following bolus administration of 0.15 IU/kg body weight of insulin lispro (Humalog, Eli Lilly, Indianapolis, IN, USA) with the study‐specific insulin pump (MiniMed 640G, Medtronic, Northridge, CA, USA). The first clamp experiment was performed with the standard infusion set (Inset II, Unomedical, Osted, Denmark) on the first day of infusion set wear to serve as baseline, while the remaining three clamp experiments were performed with the extended‐wear infusion set on Days 1, 4, and 7 of infusion set wear. The participants spent the days between the experiments (Days 2, 3, 5 and 6) at home routinely managing their diabetes while wearing the same extended‐wear infusion set in combination with the study‐specific insulin pump and the flash glucose monitor (FGM; FreeStyle Libre, Abbott Diabetes Care, Alameda, CA, USA).
2.1. Euglycaemic clamp experiment protocol
The participants were required to spend each clamp experiment fasting and on bed rest. Upon arrival at the research centre, they were fitted with the FGM (Day 1 only) and intravenous lines for frequent blood sampling and infusion of insulin and glucose. Prior to clamp start, the standard infusion set was primed with insulin lispro and inserted into the periumbilical subcutaneous adipose tissue to deliver insulin at a constant flow rate of 0.10 IU/h for the duration of the clamp. A variable intravenous infusion of human insulin (Actrapid, Novo Nordisk, Bagsvaerd, Denmark) in saline was started immediately thereafter and continued for 3 to 5 h to obtain a stable plasma glucose level of 5.6 ± 0.6 mmol/L 30 min prior to administering the subcutaneous insulin bolus (0.15 IU/kg body weight). Following bolus administration, the plasma glucose target concentration was maintained by tapering off the insulin infusion and discontinuing it when the plasma glucose dropped by 0.28 mmol/L relative to baseline. At this time, a variable intravenous glucose infusion (20% glucose; Fresenius Kabi, Bad Homburg, Germany) was started to maintain the plasma glucose target concentration for the rest of the clamp. The clamp was terminated 8 h after bolus administration or earlier if the plasma glucose level exceeded 8.3 mmol/L in the absence of glucose infusion. The plasma glucose concentration was determined at the bedside in 5–30‐min intervals using a glucose‐oxidase method (Super GL 2; Dr. Müller Gerätebau GmbH, Freital, Germany) with a coefficient of variation (CV) of less than 2%, while the plasma insulin concentration was determined as previously described, 12 using radioimmunoassay (Lispro Insulin RIA, Merck Millipore, Burlington, MA, USA) with intra‐assay CVs of 6.1%–19%.
Following the end of clamp, the participants switched to routinely managing their diabetes with the study‐specific insulin pump while being observed overnight. The described clamp experiment was repeated the next day using the extended‐wear infusion set. Upon completing the experiment, the participants were observed overnight again, and were discharged the next morning to spend the days between the remaining clamp experiments at home routinely managing their diabetes with the extended‐wear infusion set while following their daily routine. While at home, the participants documented insulin doses and carbohydrate intake in a diary and frequently monitored their glucose with the FGM system along with performing seven capillary blood glucose measurements per day using the integrated blood glucose meter. If the measured blood glucose concentration consistently exceeded 16.7 mmol/L, the participants were instructed to test the capillary blood for ketones and administer a correction bolus with an insulin pen (Humalog KwikPen). The two remaining clamp experiments were performed with the extended‐wear infusion set on Days 4 and 7 of infusion‐set wear following the described clamp protocol.
2.2. Data analysis
The data were checked for normality with the Shapiro–Wilk test. Depending on normality distribution, the variables were analysed using the mixed‐effect model, the repeated measures ANOVA, or the Friedman test. In case of a significant result (p < .05), the data were compared by means of the paired t‐test or the Wilcoxon signed rank test with an adjusted level of significance (p = .0083, for all pharmacodynamic endpoints, and the total daily insulin dose). All data are presented as median (25th–75th percentiles) or mean ± standard deviation.
3. RESULTS
3.1. Pharmacodynamics
Figure 2A shows glucose infusion rate profiles obtained following bolus administration of 0.15 IU/kg body weight of insulin lispro when using the standard and the extended‐wear infusion set on Day 1 of infusion set wear. Although time to reach the maximum glucose infusion rate (TGIRmax) was numerically considerably shorter with the standard compared with the extended‐wear infusion set (67.5 [45–115] vs. 137.5 [72.5–147.5] min; p = .046), the numerical difference did not reach statistical significance, and the total area under the glucose infusion rate curve (AUCGIR total) was similar between the sets (1042.3 [692.5–1311.5] vs. 874.2 [711.2–1130.1] mg/kg; p = .197) (Table 1).
FIGURE 2.
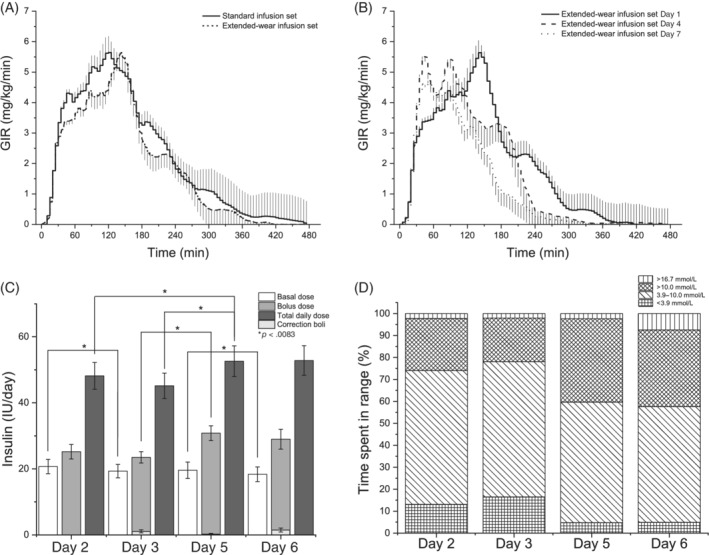
A, Glucose infusion rate (GIR) profiles (n = 16, mean ± SEM) obtained following bolus administration of 0.15 IU/kg body weight of insulin lispro when using the standard and the extended‐wear infusion set on Day 1 of infusion set wear. B, GIR profiles (n = 16, mean ± SEM) obtained following bolus administration of 0.15 IU/kg body weight of insulin lispro when using the extended‐wear infusion set on Days 1, 4 and 7 of infusion set wear. C, Total daily insulin dose (n = 13, mean ± SEM) administered at home. D, Average percentage of time spent in different glycaemic ranges at home
TABLE 1.
Summary of pharmacokinetic and pharmacodynamic variables of the standard and the extended‐wear infusion set
| Variable | Standard infusion set Day 1 Median (25th–75th) | Extended‐wear infusion set Day 1 Median (25th–75th) | p |
|---|---|---|---|
| Pharmacodynamics | |||
| GIRmax [mg/(kg*min)] | 7.3 (5.2–8.8) | 6.6 (4.1–8.4) | .482 |
| TGIRmax (min) | 67.5 (45–115) | 137.5 (72.5–147.5) | .046 |
| TGIRmax 10% (min) | 20 (15–25) | 20 (15–25) | .957 |
| TGIRmax 50% (min) | 32.5 (25–37.5) | 27.5 (22.5–45) | .971 |
| AUCGIR 0–60 min (mg/kg) | 152.9 (109.5–183) | 122.2 (84.5–172.7) | .276 |
| AUCGIR 0–120 min (mg/kg) | 396.7 (272.8–579.8) | 339.8 (250.9–458.5) | .279 |
| AUCGIR 0–240 min (mg/kg) | 809.5 (567.5–1140.5) | 734 (603.3–1009).1) | .377 |
| AUCGIR 0–360 min (mg/kg) | 1036 (692.5–1280.2) | 872.1 (709.1–1122.4) | .242 |
| AUCGIR total (mg/kg) | 1042.3 (692.5–1311.5) | 874.2 (711.2–1130.1) | .197 |
| Pharmacokinetics | |||
| TCINSmax 10% (min) | 8.2 (2.9–15.9) | 9.4 (6.2–18.1) | .052 |
| TCINSmax 50% (min) | 28.8 (23.4–35.2) | 23.3 (18.4–27.7) | .728 |
| TCINSmax (min) | 60 (40–70) | 60 (35–80) | 1.000 |
| CINSmax (pmol/L) | 461.4 (385.4–544.2) | 482.7 (384.4–561.3) | 1.000 |
| AUCINS 0‐60 min (min*pmol/L) | 15,512 (9677–18,770) | 15 153 (12,901–19,803) | 1.000 |
| AUCINS 0‐120 min (min*pmol/L) | 36,667 (29,719–46,196) | 36,944 (29,795–45,289) | 1.000 |
| AUCINS total (min*pmol/L) | 81,245 (49,308–96,847) | 69,063 (48,224–87,585) | .242 |
Abbreviations: AUCGIR 0‐tmin, area under the GIR curve from 0 min until specified time point; AUCGIR total, area under the GIR curve from 0 min until clamp end; AUCINS 0‐tmin, area under the insulin concentration curve from 0 min until specified time point; AUCINS total, area under the insulin concentration curve from 0 min until clamp end; CINSmax, maximum insulin concentration; GIRmax, maximum glucose infusion rate; TCINSmax 10%, time to reach 10% of CINSmax; TCINSmax 50%, time to reach 50% of CINSmax; TCINSmax, time to reach CINSmax; TGIRmax, time to reach GIRmax; TGIRmax 10%, time to reach 10% of GIRmax; TGIRmax 50%, time to reach 50% of GIRmax.
Figure 2B shows glucose infusion rate profiles obtained following bolus administration of 0.15 IU/kg body weight of insulin lispro when using the extended‐wear infusion set on Days 1, 4 and 7 of infusion‐set wear. While the area under the glucose infusion rate curve (AUCGIR) was comparable for the first 2 h of each clamp experiment (Day 1: 339.8 [250.9–458.5] mg/kg vs. Day 4: 458.8 [280.4–592.7] mg/kg vs. Day 7: 414.8 [228.2–540.8] mg/kg; p = .142), it significantly decreased throughout the rest of the experiment with prolonged infusion‐set wear (Day 1: 874.2 [711.2–1130.1] mg/kg vs. Day 4: 856.3 [509–1074.4] mg/kg vs. Day 7: 630.3 [328.3–814] mg/kg; p = .03). The reduction in the extent of insulin effect observed on Day 7 was accompanied by a substantial increase in the insulin absorption rate, with TGIRmax reduced by 67% compared with Day 1 (Day 1: 137.5 [72.5–147.5] vs. Day 7: 45 [35–62.5] min; p < .001). Additionally, the plasma glucose concentration variability, as expressed by the CV, did not significantly differ during the four clamp experiments (standard infusion set, Day 1: 5.7% ± 2.1%; extended‐wear infusion set, Day 1: 5.0% ± 1.1%; Day 4: 5.8% ± 1.5%; Day 7: 5.2% ± 1.4%; p = .371) (Figure 3 and Table 2).
FIGURE 3.
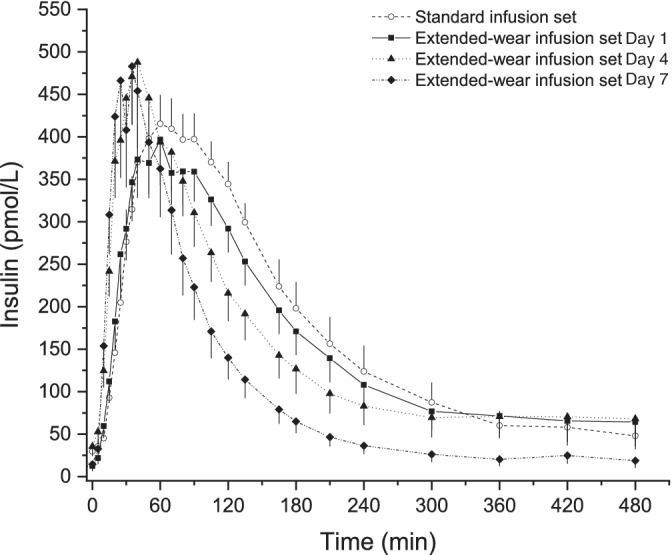
Plasma insulin concentration profiles (n = 14, mean ± SEM) obtained following bolus administration of 0.15 IU/kg body weight of insulin lispro when using the standard and the extended‐wear infusion set
TABLE 2.
Summary of pharmacokinetic and pharmacodynamic variables of the extended‐wear infusion set
| Variable | Extended‐wear infusion set Day 1 median (25th–75th) | Extended‐wear infusion set Day 4 median (25th–75th) | Extended‐wear infusion set Day 7 median (25th–75th) | p d1 vs. d4 | p d1 vs. d7 | p d4 vs. d7 |
|---|---|---|---|---|---|---|
| Pharmacodynamics | ||||||
| GIRmax [mg/(kg*min)] | 6.6 (4.1–8.4) | 7.5 (5.7–8.5) | 6.7 (4–7.2) | .151 | .595 | .065 |
| TGIRmax (min) | 137.5 (72.5–147.5) | 50 (40–80) | 45 (35–62.5) | .002 | <.001 | .215 |
| TGIRmax 10% (min) | 20 (15–25) | 20 (15–25) | 20 (15–20) | .948 | .755 | .709 |
| TGIRmax 50% (min) | 27.5 (22.5–45) | 30 (25–35) | 30 (22.5–30) | .610 | .305 | .425 |
| AUCGIR 0–60 min (mg/kg) | 122.2 (84.5–172.7) | 163.6 (136.1–210.2) | 156.6 (113.3–200.6) | .027 | .102 | .508 |
| AUCGIR 0–120 min (mg/kg) | 339.8 (250.9–458.5) | 458.8 (280.4–592.7) | 414.8 (228.2–540.8) | .060 | .891 | .223 |
| AUCGIR 0–240 min (mg/kg) | 734 (603.3–1009.1) | 815.8 (501–1017.9) | 630.3 (328.3–811) | .533 | .021 | .037 |
| AUCGIR 0–360 min (mg/kg) | 872.1 (709.1–1122.4) | 856.3 (509–1074.4) | 630.3 (328.3–814) | .173 | <.008 | .034 |
| AUCGIR total (mg/kg) | 874.2 (711.2–1130.1) | 856.3 (509–1074.4) | 630.3 (328.3–814) | .177 | <.008 | .033 |
| Pharmacokinetics | ||||||
| TCINSmax 10% (min) | 9.4 (6.2–18.1) | 4.5 (0–7.9) | 6.2 (5.8–7.2) | .052 | .052 | .052 |
| TCINSmax 50% (min) | 23.3 (18.4–27.7) | 14.9 (13.5–17.5) | 12.8 (11.6–15.5) | .011 | <.001 | .193 |
| TCINSmax (min) | 60 (35–80) | 35.5 (30–40) | 27.5 (20–35) | .243 | .016 | .000 |
| CINSmax (pmol/L) | 482.7 (384.4–561.3) | 553 (352–699) | 517.8 (290.7–763.8) | .521 | .000 | .000 |
| AUCINS 0‐60min (min*pmol/L) | 15,153 (12,901–19,803) | 21,979 (13,580–28,960) | 21,173 (10,838–30,405) | .040 | .802 | .000 |
| AUCINS 0‐120min (min*pmol/L) | 36,944 (29,795–45,289) | 40,941 (24,079–56,289) | 41,248 (14,294–48,145) | .000 | .000 | .766 |
| AUCINS total (min*pmol/L) | 69,063 (48,224–87,585) | 63,685 (43,857–86,341) | 50,770 (28,792–70,679) | .770 | .040 | .019 |
Abbreviations: AUCGIR 0‐tmin, area under the GIR curve from 0 min until specified time point; AUCGIR total, area under the GIR curve from 0 min until clamp end; AUCINS 0‐tmin, area under the insulin concentration curve from 0 min until specified time point; AUCINS total, area under the insulin concentration curve from 0 min until clamp end; CINSmax, maximum insulin concentration; GIRmax, maximum glucose infusion rate; TCINSmax 10%, time to reach 10% of CINSmax; TCINSmax 50%, time to reach 50% of CINSmax; TCINSmax, time to reach CINSmax; TGIRmax, time to reach GIRmax; TGIRmax 10%, time to reach 10% of GIRmax; TGIRmax 50%, time to reach 50% of GIRmax.
3.2. Pharmacokinetics
Both time to reach 50% of the maximum insulin concentration (TCINSmax50%) (Day 1: 23.3 [18.4–27.7] vs. Day 7: 12.8 [11.6–15.5] min; p = .011) and time to reach the maximum insulin concentration (TCINSmax) (Day 1: 60 [35–80] vs. Day 7: 27.5 [20–35] min; p = .016) were significantly shorter with prolonged infusion‐set wear. There were no statistically significant differences in the maximum insulin concentration (CINSmax) and the area under the insulin concentration curve from min 0 to min 120 (AUCINS 0–120 min) between the different study days, or between the two infusion sets, while the total area under the insulin concentration curve (AUCINStotal) was slightly but significantly reduced with prolonged infusion‐set wear (Day 1: 69,063.1 [48,223.6–87,584.9] vs. Day 7: 50,769.5 [28,791.9–70,679.4] min*pmol/L; p = .040).
The total daily insulin dose at home (Figure 2C) slightly increased over time (Day 2: 48.2 ± 14.7 vs. Day 3: 45.2 ± 13.9 vs. Day 5: 52.6 ± 16.7 vs. Day 6: 52.8 ± 16.2 IU; p = .045), while the percentage of time spent in hypoglycaemia (Figure 2D) significantly decreased (Day 2: 13.5% ± 11.3% vs. Day 3: 9.7% ± 11.2% vs. Day 5: 5.2% ± 6.8% vs. Day 6: 4.9% ± 7.8%; P = .038). The infusion set survival rate was 100% without reported infusion set failure, change of infusion site, leakage or occlusion alarms throughout the 7‐day study period. None of the participants experienced severe hypoglycaemia or ketoacidosis during the study period.
4. DISCUSSION
In this study we assessed the efficacy and safety of the novel, extended‐wear insulin infusion set featuring Lantern technology over 7 days of wear. We used the euglycaemic clamp technique to determine the pharmacokinetic and the pharmacodynamic properties of the extended‐wear infusion set in adults with type 1 diabetes following subcutaneous bolus administration of 0.15 IU/kg of rapid‐acting insulin on Days 1, 4 and 7 of infusion‐set wear. We observed a reduction in the extent of insulin absorption that was accompanied by an increase in the insulin absorption rate with prolonged infusion‐set wear.
Our findings are consistent with those of previous studies that investigated the effects of prolonged infusion‐set wear on insulin sensitivity in humans and swine. 13 , 14 The study in humans examined the pharmacodynamics of two rapid‐acting insulins over 4 days of standard infusion‐set wear and observed a significant increase in the speed of insulin absorption on the last compared with the first study day. The study in swine compared a standard with an investigational infusion set over 5 days of wear and also found that the fastest insulin absorption occurred on the last study day. In addition, histological analysis of the infusion site tissue showed that the cannula‐induced tissue damage caused by both the commercial and the investigational infusion set on Day 5 of infusion‐set wear was similar to the damage observed in clean surgical wounds. The observed reduction in the extent of insulin absorption may be offset by the accompanying increase in the insulin absorption rate. Furthermore, the increased absorption rate may also facilitate better postprandial glycaemic control (i.e. 2 h postmeal) during routine care, especially because the extent of insulin absorption observed during the postprandial time period was comparable with prolonged infusion‐set wear. Finally, although the onset of insulin action was slower with the extended wear compared with the standard infusion set at the beginning of the study, the observed difference was not significant. The initially slower onset of insulin action may be attributed to the participants being mostly on bed rest during the first two study days, because prolonged bed rest is associated with increased insulin resistance. 15
Being able to safely use the same insulin infusion set beyond the currently recommended 3 days would provide a number of benefits to individuals on CSII therapy: by changing infusion sets less frequently, the insertion‐related pain experienced may be reduced, as well as the time and effort required to change the set, which in turn may increase treatment compliance and lead to an improved quality of life. Furthermore, individuals using hybrid closed‐loop systems would presumably benefit most from the extended‐wear infusion set because, in addition to being mitigated by the faster onset of insulin action, the somewhat reduced extent of insulin absorption may also be mitigated by automated basal insulin delivery.
In summary, although this pilot study is limited because of its small population size, the non‐randomized design and the lack of a control group, the data presented suggest that the novel, extended‐wear insulin infusion set featuring Lantern technology safely maintains glycaemic control during prolonged wear.
CONFLICT OF INTEREST
PKS, MH and TS are ConvaTec employees. TRP is an advisory board member of Novo Nordisk A/S, consultant for Roche Diabetes Care, Novo Nordisk A/S, Eli Lilly & Co, Infineon, Carnegie Bank, shareholder of decide Clinical Software GmbH, and is on speaker's bureau of Novo Nordisk A/S and Astra Zeneca. JKM is an advisory board member of Abbott Diabetes Care, Boehringer Ingelheim, Becton‐Dickinson, Eli Lilly, Medtronic, Merck Sharp & Dohme GesmbH, Roche Diabetes Care and Sanofi‐Aventis GmbH, a shareholder of decide Clinical Software GmbH, and has received speaker honoraria from Abbott Diabetes Care, Astra Zeneca, Dexcom, Eli Lilly, Medtronic, Novo Nordisk A/S, Roche Diabetes Care, Sanofi‐Aventis GmbH, Servier and Takeda. The other authors declare no competing interests.
AUTHOR CONTRIBUTIONS
AS, TP and DN performed the study, interpreted data and contributed to discussions. AS, JKM and TA drafted the manuscript. WR, TRP, MH, TS and PKS contributed to discussions and interpreted data. TA, AGS and AS performed statistical analysis and interpreted data. JKM designed the study, interpreted data, contributed to discussions, supervised the project and is the guarantor of this work. All the authors critically revised and approved the final version of the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14337.
ACKNOWLEDGEMENTS
The authors would like to thank Sarah Bischof of Medical University of Graz for data monitoring and Christian Krainer of Joanneum Research for data management, as well as the participants for taking part in the study.
Simic A, Schøndorff PK, Stumpe T, et al. Survival assessment of the extended‐wear insulin infusion set featuring lantern technology in adults with type 1 diabetes by the glucose clamp technique. Diabetes Obes Metab. 2021;23:1402–1408. 10.1111/dom.14337
Funding information The study was funded by ConvaTec
DATA AVAILABILITY STATEMENT
Data are available with the authors upon request.
REFERENCES
- 1. Weissberg‐Benchell J, Antisdel‐Lomaglio J, Eshadri R. Insulin pump therapy: a meta‐analysis. Diabetes Care. 2003;26(4):1079‐1087. [DOI] [PubMed] [Google Scholar]
- 2. Pfützner A, Berger S, Spinas G. Aktueller Stellenwert der kontinuierlichen subkutanen Insulininfusion (CSII) mit Insulinpumpen in der Therapie des Diabetes mellitus. Schweiz Med Wochenschr J Suisse Med. 2000;130:1854‐1861. [PubMed] [Google Scholar]
- 3. Pickup JC, Renard E. Long‐acting insulin analogs versus insulin pump therapy for the treatment of type 1 and type 2 diabetes. Diabetes Care. 2008;31((Suppl 2)):140‐145. [DOI] [PubMed] [Google Scholar]
- 4. Berthe E, Lireux B, Coffin C, et al. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res. 2007;39(3):224‐229. [DOI] [PubMed] [Google Scholar]
- 5. Pfützner A, Sachsenheimer D, Grenningloh M, et al. Using insulin infusion sets in CSII for longer than the recommended usage time leads to a high risk for adverse events: results from a prospective randomized crossover study. J Diabetes Sci Technol. 2015;9(6):1292‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonato L, Taleb N, Gingras V, et al. Duration of catheter use in patients with diabetes using continuous subcutaneous insulin infusion: a review. Diabetes Technol Ther. 2018;20(7):506‐515. [DOI] [PubMed] [Google Scholar]
- 7. Bruttomesso D, Costa S, Baritussio A. Continuous subcutaneous insulin infusion (CSII) 30 years later: still the best option for insulin therapy. Diabetes Metab Res Rev. 2009;25(2):99‐111. [DOI] [PubMed] [Google Scholar]
- 8. Hoogma RPLM, Schumicki D. Safety of insulin glulisine when given by continuous subcutaneous infusion using an external pump in patients with type 1 diabetes. Horm Metab Res. 2006;38(6):429‐433. [DOI] [PubMed] [Google Scholar]
- 9. Renner R, Pfützner A, Trautmann M, Harzer O, Sauter K, Landgraf R. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Results of a multicenter trial. German Humalog‐CSII study group. Diabetes Care. 1999;22(5):784‐788. [DOI] [PubMed] [Google Scholar]
- 10. Hirsch IB, Bode BW, Garg S, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care. 2005;28(3):533‐538. [DOI] [PubMed] [Google Scholar]
- 11. Raskin P, Holcombe JH, Tamborlane WV, et al. A comparison of insulin lispro and buffered regular human insulin administered via continuous subcutaneous insulin infusion pump. J Diabetes Complications. 2001;15(6):295‐300. [DOI] [PubMed] [Google Scholar]
- 12. Bowsher RR, Lynch RA, Brown‐Augsburger P, et al. Sensitive RIA for the specific determination of insulin lispro. Clin Chem. 1999;45(1):104‐110. [PubMed] [Google Scholar]
- 13. Swan KL, Dziura JD, Steil GM, et al. Effect of age of infusion site and type of rapid‐acting analog on pharmacodynamic parameters of insulin boluses in youth with type 1 diabetes receiving insulin pump therapy. Diabetes Care. 2009;32(2):240‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hauzenberger JR, Hipszer BR, Loeum C, et al. Detailed analysis of insulin absorption variability and the tissue response to continuous subcutaneous insulin infusion catheter implantation in swine. Diabetes Technol Ther. 2017;19(11):641‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed‐rest‐induced insulin resistance occurs primarily in muscle. Metab ‐ Clin Exp. 1988;37(8):802‐806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available with the authors upon request.


