Scheme 1.
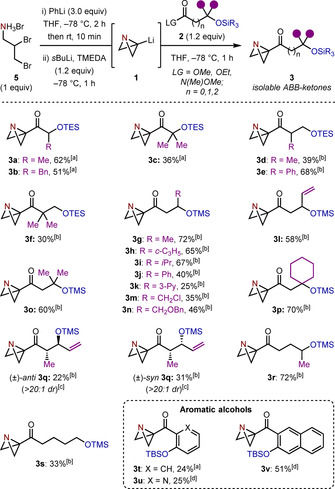
Preparation of ABB‐ketones from 1. All reactions were carried out using ammonium salt 5 (1.0 mmol) in THF (3.6 mL), trimethylsilyl (TMS), triethylsilyl (TES) and tert‐butyldimethylsilyl (TBS) ethers, 2, were added as 1.0 M solutions in THF. Isolated yields given. [a] From the corresponding ethyl ester. [b] From the corresponding Weinreb amide. [c] The diastereomeric ratio (dr) was determined by 1H NMR analysis of the crude reaction mixture. The dr of Weinreb amides was >20:1 [d] From the corresponding methyl ester.
