Table 1.
Optimization of the reaction conditions.
|
Entry[a] |
1 a (equiv) |
Ligand (mol %) |
Base (equiv) |
Additive (equiv) |
Yield [%][b] |
|---|---|---|---|---|---|
|
1[c] |
1.2 |
– |
KOtBu (1.2) |
– |
43 |
|
2 |
1.2 |
L1 (20) |
KOtBu (1.2) |
– |
47 |
|
3 |
1.2 |
L1 (20) |
KOtBu (1.5) |
– |
53 |
|
4 |
1.2 |
L1 (20) |
KOtBu (2) |
– |
62 |
|
5 |
1.2 |
L1 (20) |
KOtBu (2.5) |
– |
54 |
|
6 |
1.2 |
L1 (20) |
KOtBu (2) |
LiCl (1.2) |
75 |
|
7 |
1.5 |
L1 (20) |
KOtBu (2) |
LiCl (1.2) |
83 |
|
8 |
2 |
L1 (20) |
KOtBu (2) |
LiCl (1.2) |
89 |
|
9 |
2.5 |
L1 (20) |
KOtBu (2) |
LiCl (1.2) |
95 |
|
10 |
3 |
L1 (20) |
KOtBu (2) |
LiCl (1.2) |
95 |
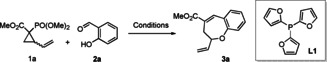
[a] Reaction conditions: 2 a (0.236 mmol), Pd2dba3 (5 mol %), L1 (20 mol %), 1.18 mL of THF (1.18 mL), 50 °C, overnight. [b] Determined by 1H NMR analysis using 2,5‐dimethylfuran as an internal standard. [c] Pd(PPh3)4 was used instead of Pd2dba3 and L1. dba=dibenzylideneacetone.
