Table 2.
Optimization of the asymmetric synthesis of 3 a.
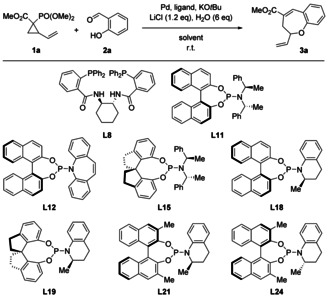
|
Entry[a] |
Ligand |
Solvent |
Yield [%][b] |
e.r.[c] |
|---|---|---|---|---|
|
1 |
L8 |
THF |
15 |
64:36 |
|
2 |
L11 |
THF |
30 |
51:49 |
|
3 |
L12 |
THF |
31 |
50:50 |
|
4 |
L15 |
THF |
40 |
73:27 |
|
5 |
L18 |
THF |
79 |
56:44 |
|
6 |
L19 |
THF |
20 |
76:24 |
|
7 |
L21 |
THF |
15 |
66:34 |
|
8 |
L24 |
THF |
98 |
77:23 |
|
9[d] |
L24 |
THF |
99 |
80:20 |
|
10[d,e] |
L24 |
THF |
98 |
80:20 |
[a] Reaction conditions: 2 a (0.236 mmol), Pd2dba3 (5 mol %), ligand (10 mol % for bidentate, 20 mol % for monodentate), 1 a (0.590 mmol), LiCl (0.283 mmol), H2O (26 μL), KOtBu (0.472 mmol), solvent (1.18 mL), room temperature, overnight. [b] Determined by 1H NMR analysis using 2,5‐dimethylfuran as an internal standard. [c] Determined by SFC analysis on a chiral stationary phase. [d] 1 a: 0.826 mmol. [e] PdCl2 (10 mol %) and L24 (40 mol %) were used instead of Pd2dba3.
