Scheme 2.
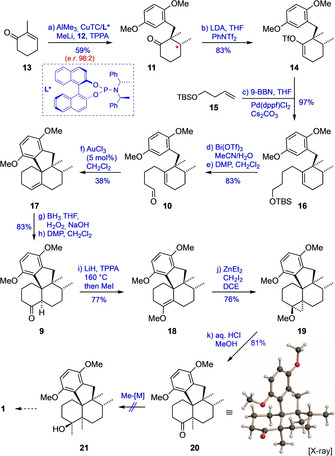
Synthesis of key intermediate 9 and unsuccessful attempts at its conversion to 1. Reagents and conditions: a) AlMe3, CuTC/L1* (2.4 mol %), Et2O, −30 °C, 4.5 h; then MeLi, 12, TPPA, −30→25 °C, 17 h; b) LDA, THF, PhNTf2, −78→25 °C, 3 h; c) 15, 9‐BBN, THF, 25 °C, 2 h; then 14, Pd(dppf)Cl2 (3 mol %), Cs2CO3, H2O, DMF, 25 °C, 1 h; d) Bi(OTf)3 (4 mol %), CH3CN/H2O, 25 °C, 1.5 h, 97 %; e) DMP, CH2Cl2, 0→25 °C, 2 h, 86 %; f) AuCl3 (5 mol %), CH2Cl2, 0 °C, 11 min; g) BH3 ⋅THF, THF, 0→30 °C, 10 h; then NaOH, H2O2, THF/H2O, 0→25 °C, 14 h; h) DMP, CH2Cl2, 0→30 °C, 2 h; i) LiH, TPPA, 160 °C, 1.5 h; then MeI, 25 °C, 20 h; j) ZnEt2, CH2I2, DCE, 25 °C, 35 min; k) aq. HCl, MeOH, reflux, 35 min. CuTC=copper(I) thiophene‐2‐carboxylate, TPPA=tripyrrolidinophosphoric acid triamide, LDA=lithium diisopropylamide, TBS=tert‐butyldimethylsilyl, 9‐BBN=9‐borabicyclo[3.3.1]nonane, dppf=1,1′‐bis(diphenylphosphino)ferrocene, DMP=Dess–Martin periodinane, DCE=dichloroethane.
