Figure 1.
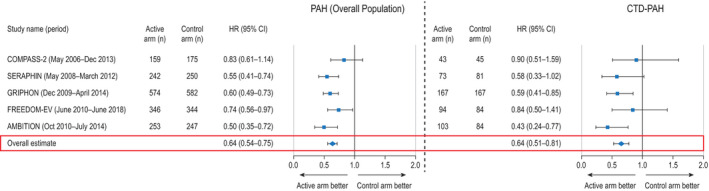
Time to clinical morbidity/mortality event for all patients with pulmonary arterial hypertension (PAH) (left) and patients with connective tissue disease (CTD)–associated PAH (right) in randomized, controlled trials that evaluated time to clinical morbidity/mortality event as a primary end point (5 trials). Results are depicted as forest plots, showing the hazard ratio (HR) with 95% confidence interval (95% CI) in the active treatment group relative to the control group. Overall HRs were estimated using random‐effects models. COMPASS‐2 = Combination of Bosentan and Sildenafil Versus Sildenafil Monotherapy on PAH; SERAPHIN = Study with an Endothelin Receptor Antagonist in PAH to Improve Clinical Outcome; GRIPHON = Prostacyclin Receptor Agonist (Prostaglandin I2) in PAH; FREEDOM‐EV = International, Multicenter, Randomized, Double‐blind, Placebo‐controlled Event‐driven Trial of Oral Treprostinil in Subjects with PAH; AMBITION = Ambrisentan plus Tadalafil in PAH.
