Figure 2.
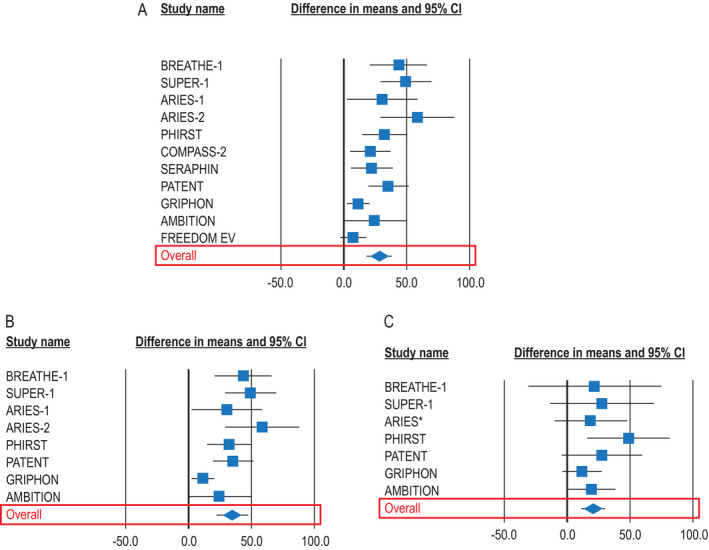
Change in the 6‐minute walk distance (6MWD) for all patients with pulmonary arterial hypertension (PAH) of any etiology in all randomized, controlled trials (RCTs) (11 trials) (A), for all patients in RCTs that reported 6MWD in patients with connective tissue disease (CTD)–associated PAH (8 trials) (B), and for patients with CTD‐PAH (8 trials) (C). Results are depicted as forest plots, showing the mean change in the 6MWD from baseline to between 3 and 6 months, with 95% confidence interval (95% CI). *Combined data from the Randomized, Double‐blind, Placebo‐controlled, Multicenter, Efficacy Study of Ambrisentan for PAH 1 and 2 (ARIES‐1 and ARIES‐2, respectively). BREATHE‐1 = Bosentan Randomized Trial of Endothelin Antagonist Therapy; SUPER‐1 = Sildenafil Use in PAH; PHIRST = PAH and Response to Tadalafil; COMPASS‐2 = Combination of Bosentan and Sildenafil Versus Sildenafil Monotherapy on PAH; SERAPHIN = Study with an Endothelin Receptor Antagonist in PAH to Improve Clinical Outcome; PATENT = PAH Soluble Guanylate Cyclase–Stimulator Trial 1; GRIPHON = Prostacyclin Receptor Agonist (Prostaglandin I2) in PAH; AMBITION = Ambrisentan plus Tadalafil in PAH; FREEDOM‐EV = International, Multicenter, Randomized, Double‐blind, Placebo‐controlled Event‐driven Trial of Oral Treprostinil in Subjects with PAH.
