Scheme 3.
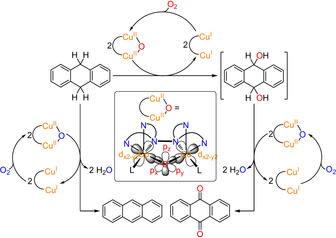
Reactivity studies and proposed mechanism for the oxygenation of DHA. Reaction of DHA in the presence of the Cu2O catalyst leads to anthraquinone (AQ) via a disecondary alcohol (9,10‐dihydroxy‐9,10‐dihydroanthracene) which is formed as primary oxygenation product. The subsequent oxidation may also be catalyzed by the Cu2O species (right). As a byproduct, anthracene is formed by twofold H‐atom abstraction proceeding without O‐transfer (left). In all reactions the formed CuI−CuI species are re‐oxidized to the Cu2O complex with O2, following the pathway of Scheme 2. In contrast to the actual monooxygenation reaction (top), both the twofold two‐electron oxidation of the disecondary alcohol and the double HAT generate water which may deactivate the catalyst. Inset: Simplified structure of the Cu2O complex showing the orbitals involved in the substrate oxygenation, i.e., the d orbitals of the copper centers and the px, py and pz orbitals of the oxo ligand. L=coordinating solvent molecule (C3H6O).
