Figure 1.
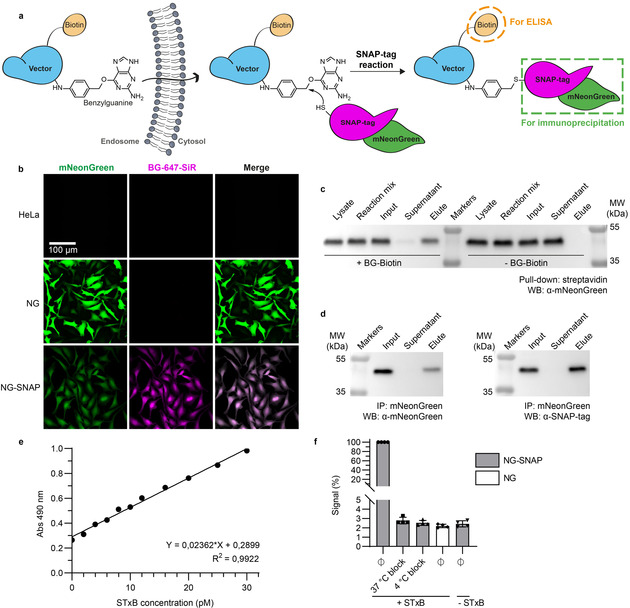
A robust, sensitive, and quantitative cytosolic arrival assay. a) Schematic representation of the Cyto‐SNAP assay. Upon membrane translocation, the BG‐modified vector encounters the cytosolic mNeonGreen‐SNAP‐tag protein with which it reacts covalently. mNeonGreen is exploited for immunoprecipitation on beads coated with anti‐mNeonGreen nanobodies, and the biotin moiety for ELISA. b) Parental, polyclonal mNeonGreen expressing (NG) or monoclonal mNeonGreen‐SNAP‐tag expressing (NG‐SNAP) HeLa cell lines were treated with fluorescent SNAP‐tag ligand BG‐647‐SiR. SiR fluorescence was only observed on NG‐SNAP cells, in which it was homogeneously distributed in the cytosolic space. c) Demonstration of the high efficiency of the SNAP‐tag reaction. Lysate from NG‐SNAP cells was incubated with excess benzylguanine–biotin (BG–biotin) ligand, followed by streptavidin pull‐down. Western blotting analysis showed that the cell lysate was depleted of mNeonGreen‐SNAP‐tag protein, which was indeed recovered on beads. d) Demonstration that the mNeonGreen immunoprecipitation (IP) is complete. The mNeonGreen‐SNAP‐tag protein was totally recovered on mNeonGreen‐Trap beads, which confirmed the efficacy of the immunoprecipitation. e) Sensitivity and linearity of the assay. Known amounts of STxB‐BG‐biotin conjugate C were added into NG‐SNAP cell lysate, followed by incubation at 37 °C, mNeonGreen‐SNAP‐tag immunoprecipitation and ELISA development. The obtained standard curve was linear over a wide range of concentrations. Even low picomolar STxB‐BG‐biotin concentrations were robustly detected. f) Demonstration that non‐reacted SNAP‐tag protein is efficiently quenched before cell lysis. Intact NG‐SNAP and NG cells were incubated for 30 min at 4 °C or 37 °C in presence or absence (Ø) of SNAP‐Cell® Block reagent. The cells were then washed, lysed, and lysates were incubated at 4 °C with or without STxB‐BG‐biotin conjugate A. Both 37 °C and 4 °C block conditions gave ELISA signals comparable to background signal without STxB‐BG‐biotin incubation.
