FIGURE 2.
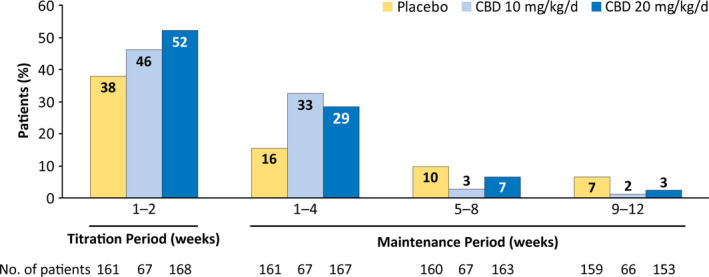
Adverse events by time of onset. If a patient had multiple occurrences of an adverse event (AE), then the AE was counted once for the first occurrence only. Percentage of patients was calculated based on the number of patients in the safety analysis set who had a visit or follow‐up call within each time period. One patient in the placebo group first experienced an AE after the 14‐week treatment period. CBD, cannabidiol
