Summary
Background
Ginger is a spice with a long history of use as a traditional remedy for nausea and vomiting. No data on the efficacy of ginger are presently available for children with vomiting associated with acute gastroenteritis (AGE).
Aim
To test whether ginger can reduce vomiting in children with AGE.
Methods
Double‐blind, randomised placebo‐controlled trial in outpatients aged 1 to 10 years with AGE‐associated vomiting randomised to ginger or placebo. The primary outcome was the occurrence of ≥1 episode of vomiting after the first dose of treatment. Severity of vomiting and safety were also assessed.
Results
Seventy‐five children were randomised to the ginger arm and 75 to the placebo arm. Five children in the ginger arm and 4 in the placebo arm refused to participate in the study shortly after randomisation, leaving 70 children in the ginger arm and 71 in the placebo arm (N = 141). At intention‐to‐treat analysis (N = 150), assuming that all children lost to follow‐up had reached the primary outcome, the incidence of the main outcome was 67% (95% CI 56 to 77) in the ginger group and 87% (95% CI 79 to 94) in the placebo group, corresponding to the absolute risk reduction for the ginger versus the placebo group of −20% (95% CI −33% to −7%, P = 0.003), with a number needed to treat of 5 (95% CI 3 to 15).
Conclusion
Oral administration of ginger is effective and safe at improving vomiting in children with AGE.
Trial registration: The trial was registered on https://clinicaltrials.gov/ with the identifier NCT02701491.
In a double‐blind, randomised placebo‐controlled trial in pediatric outpatients with acute gastroenteritis (AGE)‐associated vomiting the oral administration of ginger resulted effective and safe at improving vomiting.

1. INTRODUCTION
Vomiting is a common symptom in childhood and has many causes, ranging from self‐limited to life‐threatening conditions. 1 Vomiting is the presenting symptom in up to 75% of children with acute gastroenteritis (AGE), where it contributes to fluid loss, failure of oral rehydration therapy, and emergency admission to the hospital. 2 , 3 Nearly 80% of Italian pediatricians prescribe antiemetic drugs to children with AGE, mostly off‐label. 4 , 5 , 6 The antiemetic drugs most frequently prescribed in Europe and Italy are domperidone, a dopamine receptor antagonist, and ondansetron, a 5‐HT3 antagonist. 6 Current evidence shows that ondansetron but not domperidone is effective for the treatment of AGE‐associated vomiting in the emergency setting. 2 , 7
Ginger (Zingiber officinale) is a spice with a long history of use as traditional remedy for nausea and vomiting. 8 The active phenolic compounds of ginger, that is, gingerols, zingiberene and shogaols, have also anti‐inflammatory and anti‐oxidant properties. The antiemetic action of ginger has been investigated in various conditions including motion sickness, pregnancy, post‐anesthesia, post‐surgery, and chemotherapy‐induced nausea and vomiting. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Given at doses up to 2 g/day, ginger is effective at controlling vomiting without side effects. Besides its general anti‐inflammatory action, 26 , 27 potential mechanisms of action of ginger include the inhibition of 5‐HT3 and muscarinic acetylcholine (M3) receptors, and the modulation of esophageal and gastrointestinal motility. 17 , 26 , 28
The pharmacological properties of ginger have been investigated mostly in adults, and no data are available on its effects in children. However, many ginger‐based food supplements are increasingly available on the market and are used for the prevention and treatment of vomiting in children without any proof of efficacy. Therefore, the present randomised trial was designed to test whether ginger can reduce AGE‐associated vomiting in children.
2. METHODS
2.1. Trial design
This randomised, double‐blind, parallel‐arm, placebo‐controlled trial was performed in collaboration with family pediatricians of the Naples city area working for the Italian National Health System and was coordinated by the Department of Translational Medical Science of the University Federico II. The trial was approved by the Ethics Committee of University Federico II of Naples and was performed in accordance with the Helsinki Declaration (Tokyo revision, 2004), and with the pertinent European and Italian regulations about privacy. Written informed consent to participate in the study was obtained by the parents of the children. The trial was registered on https://clinicaltrials.gov/ with the identifier NCT02701491. Such registration was performed before the enrollment of the first patient.
2.2. Participants
The inclusion criteria were age between 1 and 10 years; suspected AGE‐related symptoms lasting <12 h: AGE‐associated vomiting (not bilious or bloody) from <4 h; modification of stool pattern lasting <12 h; mild to moderate dehydration evaluated as described elsewhere. 29
The exclusion criteria were concomitant presence of other diseases, including neurologic and neuropsychiatric diseases; genetic and metabolic diseases, autoimmune diseases, immunodeficiencies, celiac disease, cancer, adverse food reactions (including ginger allergy); functional gastrointestinal disorders; inflammatory bowel diseases; liver diseases; pancreatic diseases; malformations of the gastrointestinal tract; infectious diseases other than AGE; severe dehydration; malnutrition defined as weight‐for‐height <3 standard deviation scores (SDS); previous surgery of the respiratory, gastrointestinal or urinary tract; use of gastric acidity inhibitors, antibiotics, antiemetics or other drugs in the 2 weeks before the enrollment; use of prebiotics, probiotics or symbiotics in the 2 weeks before the enrollment; participation to other studies.
We evaluated socioeconomic status according to the Hollingshead Four Factor Index for Socioeconomic Status, which is a validated tool to assess this variable. 30
The final diagnosis of AGE was reached in the presence of ≥3 bowel movements of soft or liquid stools over 24 h, with or without fever, following the guidelines of the European Society for Pediatric Gastroenterology Hepatology and Nutrition and of the European Society for Pediatric Infectious Diseases. 31 Microbiological and other laboratory investigations were performed only for specific clinical reasons.
2.3. Intervention
The placebo and ginger products were in liquid form and their composition is given in Table 1. Products were produced under Good Manufacturing Practice by the Laboratory of Budetta Farma (Montecorvino Pugliano, Italy). Only a single batch of both ginger and placebo were used in the whole study. The distribution of the treatments was carried out by the coordinator center (Department of Translational Medical Science at the University Federico II, Naples, Italy). The packaging, color, weight, smell and taste of the ginger and placebo were similar. The similar smell and taste were obtained by adding anise and aromas to both products. The first dose of treatment (20 drops containing 10 mg of product) was administered by the family pediatricians immediately after the enrollment of the children and was followed by the administration of hypotonic oral rehydration solution (ORS) after 30 min. 31 The parents were instructed to administer 20 additional drops of treatment every 8 h after the first dose, until the resolution of vomiting. Additional doses were provided only in the presence of ≥1 episode of vomiting in the previous 8 h. The parents received a glass bottle with a pipette and a recyclable cardboard case and were instructed by their family pediatricians on how to use it. In addition, the parents were given a daily diary and were instructed by the family pediatricians on how to compile it. The diary recorded: quantity of ORS; whether ORS was refused; number of episodes of vomiting; presence of diarrhea or abdominal pain; number of bowel movements; stool consistency (Bristol stool scale); presence of systemic symptoms such as fever, headache, and irritability; suspected adverse reactions; hospitalisations; use of intravenous fluid therapy; number of days of school lost by the children; number of days of work lost by the parents. A complete medical examination was performed every 24 h by the family pediatricians until the disappearance of all AGE‐related symptoms. Unscheduled visits were performed if necessary.
TABLE 1.
Composition of ginger and placebo
|
Placebo (% of weight) |
Ginger (% of weight) |
|
|---|---|---|
| Water | 48.37 | 47.37 |
| Fructose | 40 | 40 |
| Sodium citrate | 5 | 5 |
| Anise | 0.02 | 0.02 |
| Vitamin B1 | 0.03667 | 0.03667 |
| Vitamin B6 | 0.04665 | 0.04665 |
| Vitamin B2 | 0.04668 | 0.04668 |
| L‐alanine | 0.25 | 0.25 |
| Potassium citrate | 4.25 | 4.25 |
| Citric acid | 1.75 | 1.75 |
| Aroma | 0.18 | 0.18 |
| Potassium sorbate | 0.03 | 0.03 |
| Stevia Rebaudiana | 0.02 | 0.02 |
| Zingiber officinale hydroglycerin extract | — | 1 |
| Total | 100 | 100 |
20 drops dose contained 10 mg of ginger.
2.4. Outcomes
The primary outcome was the occurrence of ≥1 episode of vomiting after the first dose of ginger or placebo administered by the family pediatricians. The secondary outcomes were the incidence and the number of episodes of vomiting 24 h (day 1) and 48 h (day 2) after the first dose of treatment. Other outcomes were the quantity of ORS taken by the children in the 4 h after the first dose of treatment; the number of children refusing ORS; the number of children with diarrhea at 24 h (day 1), 48 h (day 2) and 72 h (day 3) from the first dose of treatment; the number of children requiring intravenous fluid rehydratation; the number of children requiring hospitalisation; the number of children not attending school at 24 h (day 1), 48 h (day 2) and 72 h (day 3) from the first dose of treatment.
2.5. Sample size calculation
Seventy‐three subjects per arm were needed to detect an absolute difference of 20% (from 35% to 15%) in the occurrence of ≥1 episode of vomiting after the first dose of treatment between the placebo arm and the ginger at an alpha level of 0.05 with a power of 0.80 (Pearson's chi‐squared test) (Stata 14.0, Stata Corp, College Station, TX, US). Such difference was considered clinically relevant in another study. 29 Estimating a dropout rate <3% basing on the results of a previous study, 29 we enrolled 75 subjects per arm, for a total of 150 subjects.
2.6. Randomisation
The family pediatricians administered the treatment according to a computer‐generated stratified randomisation list produced using the ralloc command 32 (Stata 14.0, Stata Corp, College Station, TX, USA). The randomisation list employed 5 strata, 1 for each family pediatrician involved into the study, and block sizes of 2. Thus, each pediatrician had her/his own randomisation list involving 30 children randomly assigned to ginger or placebo in 1:1 ratio using block sizes of 2.
2.7. Allocation concealment
Ginger and placebo were packaged in glass bottles and consecutively numbered according to the randomisation list generated for each family pediatrician (see Section 2.6.).
2.8. Blinding
The family pediatricians (assessors of the primary outcome), the children and their parents (assessors of the secondary and other outcomes), and the researchers who performed data entry, were blinded to the treatment. The statistician who performed the analysis was not blinded to the treatment.
2.9. Data collection
Study monitoring was performed by an independent clinical trial monitor and included on‐site visits and telephone interviews with family pediatricians. The clinical trial monitor reviewed the clinical forms for completeness, clarity, and consistency. All the data were recorded anonymously and entered into the study database by the same researcher. The study database underwent data cleaning according to standard procedures and was locked before statistical analysis.
2.10. Compliance
To assess compliance to the treatment, the parents were asked to return the bottles containing the treatment. Compliance to the treatment was defined as the consumption of 100% of it.
2.11. Statistical analysis
Most continuous variables had non‐Gaussian distributions, and all are reported as medians and interquartile ranges (IQR). Discrete variables are reported as numbers and proportions. The SDS of weight, height, and body mass index (BMI) were calculated using the World Health Organisation reference data. 33
The primary outcome, that is, the occurrence of ≥1 episode of vomiting after the first dose of treatment, was evaluated using a binomial regression model. The response variable of the model was the occurrence of ≥1 episode of vomiting after the first dose of treatment (discrete: 0 = no; 1 = yes) and the predictor variable was treatment (discrete: 0 = placebo; 1 = ginger). The point estimate and the 95% confidence interval (95% CI) of the absolute risk reduction (ARR) were obtained from the model. 34 The 95% CI of the number needed to treat (NNT), that is, the number of patients to treat to prevent the occurrence of ≥1 episode of vomiting after the first dose of treatment, was calculated using Bender's formula. 35 We performed an intention‐to‐treat analysis (ITT) of the primary outcome by considering the children lost after randomisation as follows: (1) all missing values of the primary outcome set to the worst outcome in both the ginger and placebo arms (equal‐case scenario ITT) and (2) missing values of the primary outcome set to the worst outcome in the ginger arm and to the best outcome in the placebo arm (worst‐case scenario ITT). 36 The worst outcome was defined as the occurrence of ≥1 episode of vomiting after the first dose of treatment; the best outcome was its opposite. The equal‐case scenario ITT for the primary outcome was prespecified by the study protocol, as per standard practice. The worst‐case scenario ITT for the primary outcome was implemented post hoc.
The secondary outcomes, which involve repeated measures, were evaluated using per‐protocol analysis. The incidence rate of vomiting after 24 h (day 1) and 48 h (day 2) from the first dose of treatment was evaluated using a binomial regression model for repeated measures. 34 , 37 The response variable of the model was the incidence of vomiting (discrete: 0 = no; 1 = yes), and the predictors were treatment (discrete: 0 = placebo; 1 = ginger), time (discrete: 0 = day 1; 1 = day 2), and a treatment × time (discrete × discrete) interaction. Repeated measures were taken into account by using subject‐specific cluster confidence intervals. Two prespecified between‐group (ginger vs. placebo) within‐day (day 1 and day 2) contrasts were used to calculate the time‐specific ARR. ARR and p values were corrected using a Bonferroni correction for two contrasts (day 1 and day 2).
The number of episodes of vomiting were calculated using a negative binomial regression model for repeated measures. 34 , 37 The response variable of the model was the number of episodes of vomiting (count) and the predictors were treatment (discrete: 0 = placebo; 1 = ginger), time (discrete: 0 = day 1; 1 = day 2), and a treatment × time (discrete × discrete) interaction. Repeated measures were taken into account by using subject‐specific cluster confidence intervals. Two prespecified between‐group (ginger vs. placebo) within‐day (day1 and day 2) contrasts were used to calculate the time‐specific ARR. ARR and P values were corrected using a Bonferroni correction for two contrasts (day 1 and day 2).
The remaining outcomes were reported only as descriptive (and not inferential) statistics, using medians and interquartile ranges (IQR) for continuous variables and numbers and proportions for discrete variables. Such outcomes were: quantity of ORS assumed by the children in the 4 h after the first dose of treatment; number of children refusing ORS; number of children with diarrhea after 24 h, 48 h and 72 h from the first dose of treatment; number of children requiring intravenous fluid therapy; number of children requiring hospitalization; number of children not attending school after 24 h, 48 h and 72 h from the first dose of treatment.
Statistical analysis was performed using Stata 16.1 (Stata Corporation, College Station, TX, USA).
3. RESULTS
3.1. Flow diagram
The trial was performed between March 2016 and April 2017. The flow diagram of the trial is depicted in Figure 1. A total of 168 children were assessed for eligibility and 18 were excluded, 11 because they did not meet the inclusion criteria, and 7 because their parents declined to participate in the study. The remaining 150 children were randomised in a 1:1 ratio to ginger (n = 75) and to placebo (n = 75). Five children in the ginger arm and 4 in the placebo arm abandoned the study shortly after randomisation, leaving 70 children in the ginger arm and 71 children in the placebo arm.
FIGURE 1.
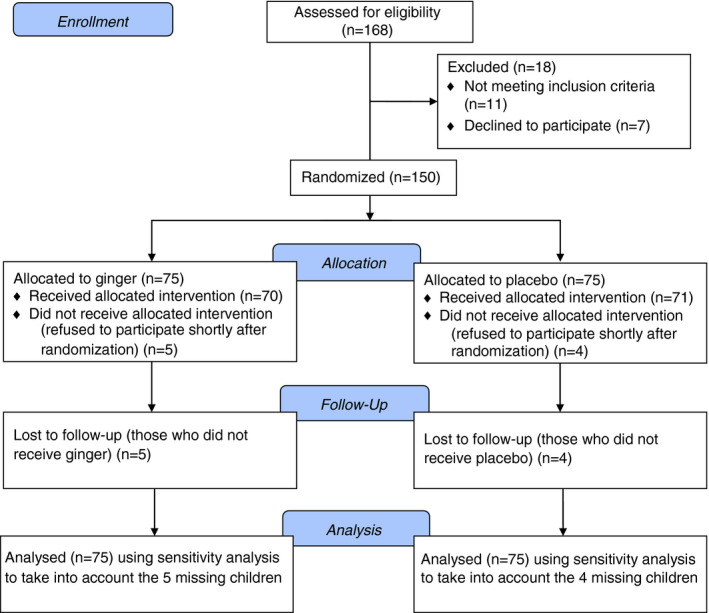
Flow of the children through the study
3.2. Baseline data
Table 2 shows that the children randomised to placebo and ginger had similar baseline features. All children were from families of middle socioeconomic status and lived in the city area of Naples. The vaccination status was similar in the two groups and no child had received anti‐Rotavirus or anti‐Influenza vaccination (data not shown).
TABLE 2.
Baseline features of the placebo and ginger arms
|
Placebo (n = 75) |
Ginger (n = 75) |
|
|---|---|---|
| Male gender, n (%) | 36 (48) | 39 (52) |
| Age (years) | 6 (4; 9) | 5 (3; 7) |
| Cesarean delivery, n (%) | 44 (59) | 49 (65) |
| Gestational age (weeks) | 39 (38; 40) | 39 (38; 40) |
| Breastfeeding, n (%) | 47 (63) | 40 (53) |
| Breastfeeding duration (months) | 6 (3; 9) | 4 (3; 7) |
| Age at weaning (months) | 4 (4; 5) | 4 (4; 5) |
| Vomiting (episodes in the previous 4 h) | 4 (3; 5) | 4 (3; 5) |
| Diarrhea (bowel movements in the previous 4 h) | 1 (1; 1) | 1 (1; 2) |
| Body weight (kg) | 23 (15; 31) | 19 (15; 27) |
| Body weight (SDS WHO) | 0.55 (0.01; 0.94) | 0.66 (−0.08; 1.15) |
| Height (m) | 1.16 (1.03; 1.31) | 1.06 (0.98; 1.25) |
| Height (SDS WHO) | −0.08 (−0.41; 0.19) | 0.16 (−0.28; 0.46) |
| BMI (kg/m2) | 17 (16; 19) | 17 (16; 19) |
| BMI (SDS WHO) | 0.81 (0.26; 1.33) | 0.83 (0.02; 1.54) |
Continuous variables are reported as median (50th percentile) and interquartile range (IQR, 25th and 75th percentiles). Discrete variables are reported as the number and proportion of subjects with the characteristic of interest.
Abbreviations: BMI, body mass index; SDS, standard deviation scores; WHO, World Health Organization.
3.3. Primary outcome
The primary outcome, that is, the occurrence of ≥1 episode of vomiting after the first dose of treatment, is reported in Table 3.
TABLE 3.
Incidence of the primary outcome in the ginger and placebo arms
|
ITT equal case scenario |
ITT worst‐case scenario |
PPA | |
|---|---|---|---|
| Placebo event rate placebo |
65/75 0.87 [0.79 to 0.94] |
61/75 0.81 [0.73 to 0.90] |
61/71 0.86 [0.78 to 0.94] |
| Ginger event rate ginger |
50/75 0.67 [0.56 to 0.77] |
50/75 0.67 [0.56 to 0.77] |
45/70 0.64 [0.53 to 0.76] |
| Absolute risk reduction (ginger‐placebo) |
−0.20 a [−0.33 to −0.07] P = 0.003 (Wald) |
−15 a [−0.29 to −0.006] P = 0.038 (Wald) |
−0.22 a [−0.35 to −0.08] P = 0.002 (Wald) |
| Number needed to treat | 5 b [3 to 15] | 7 b [4 to 167] | 5 b [3 to 14] |
| Number of children | 150 | 150 | 141 |
Values are proportions and 95% confidence interval (in square brackets) from binomial regression. The ITT “equal case scenario” analysis assumes the occurrence of the best outcome (no vomiting) in the 5 children lost to follow‐up in the ginger arm and of the same outcome (no vomiting) in the 4 children lost to follow‐up in the placebo arm. The ITT “worst case scenario” analysis assumes the occurrence of the worst outcome (vomiting) in the 5 children lost to follow‐up in the ginger arm and of the best outcome (no vomiting) in the 4 children lost to follow‐up in the placebo arm.
Abbreviations: ITT, intention‐to treat analysis; PPA, per‐protocol analysis.
95 confidence interval calculated from binomial regression.
95 confidence interval calculated from Bender's formula.
Assuming under ITT that all children lost to follow‐up had reached the primary outcome, the ARR was −20% (−33% to −7%, P = 0.003, N = 150). Under the worst‐case scenario ITT analysis, that is, assuming that the 4 children lost in the placebo arm had not vomiting and the 5 children lost in the ginger arm had vomiting, the ARR was −0.15 (95% CI −0.29 to −0.006, P = 0. 038, N = 150) for ginger versus placebo. Ignoring children lost to follow‐up, that is, performing a per‐protocol analysis, the ARR was −22% (−35% to −8%, P = 0.002, N = 141).
3.4. Secondary outcomes
Table 4 gives the incidence of vomiting at 24 h and 48 h from the first dose of treatment (per‐protocol analysis, binomial regression). The ARR in the incidence of vomiting for the ginger versus the placebo arm was −22% (P = 0.005) at day 1 and −19% (P = 0.04) at day 2.
TABLE 4.
Incidence of vomiting 24 and 48 h after the first dose of treatment (secondary outcome, per‐protocol analysis)
| 24 h after the first dose of treatment | 48 h after the first dose of treatment | |
|---|---|---|
|
Placebo event rate |
61/71 0.86 [0.78 to 0.94] |
41/71 0.58 [0.46 to 0.69] |
| Ginger event rate |
45/70 0.64 [0.53 to 0.76] |
27/70 0.39 [0.27 to 0.50] |
|
Absolute risk reduction (ginger‐placebo) |
−0.22 [−0.38 to −0.06] a P = 0.005 (Wald) b |
−0.19 [−0.38 to −0.01] a |
Values are proportions and 95% confidence intervals (in square brackets) from binomial regression.
Bonferroni's corrected 95% confidence interval (correction for two comparisons).
Bonferroni's corrected p value (correction for two comparisons).
Table 5 gives the number of episodes of vomiting at 24 h and 48 h after the first dose of treatment (per‐protocol analysis, negative binomial regression). The difference in the number of episodes of vomiting for the ginger versus the placebo arm was −0.54 (P = 0.08) at day 1 and −0.64 (P = 0.003) at day 2.
TABLE 5.
Number of episodes of vomiting 24 and 48 h after the first dose of treatment (secondary outcome, per‐protocol analysis)
| 24 h after the first dose of treatment | 48 h after the first dose of treatment | |
|---|---|---|
| Number of episodes of vomiting—placebo | 2.03 [1.67 to 2.39] | 1.18 [0.85 to 1.52] |
|
Number of episodes of vomiting—ginger |
1.49 [1.12 to 1.86] | 0.54 [0.33 to 0.75] |
| Difference in the number of episodes of vomiting (ginger‐placebo) |
−0.54 [−1.13 to 0.05] a P = 0.08 (Wald) b |
−0.64 [−1.09 to −0.19] a P = 0.003 (Wald) a |
Values are means and 95% confidence intervals (in square brackets) calculated from negative binomial regression.
Bonferroni's corrected 95% confidence interval (correction for two comparisons).
Bonferroni's corrected P value (correction for two comparisons).
3.5. Other outcomes
The other outcomes were analysed using per‐protocol analysis and are reported as descriptive statistics only. The median (IQR) volume of ORS assumed by the children in the 4 h after the first dose of treatment was 30 (15; 40) ml/kg in the ginger arm and 10 (5; 25) ml/kg in the placebo arm. The number of children refusing ORS was 17 (24%) in the ginger arm and 37 (52%) in the placebo arm. The number of children with diarrhea after 24 h, 48 h and 72 h from the first dose of treatment was 38 (54%), 30 (43%) and 7 (10%) in the ginger arm, and 36 (51%), 30 (42%), 13 (18%) in the placebo arm. Intravenous fluid rehydration or hospitalisation were not required for any child. The number of children not attending school 24 h, 48 h and 72 h after the first dose of treatment was 39 (56%), 31 (44%) and 20 (29%) in the ginger arm, and 57 (80%), 40 (56%) and 21 (30%) in the placebo arm.
3.6. Compliance
The intervention was well accepted by the children as confirmed by an adherence rate of 100%.
3.7. Adverse effects
There were no reported adverse effects attributable placebo or ginger.
4. DISCUSSION
To the best of our knowledge, this is the first RCT aimed at evaluating the antiemetic effect of ginger at reducing vomiting in children with AGE. We found that, even under the worst‐case scenario ITT, ginger was able to reduce AGE‐associated vomiting.
The present study has several strengths. The main strength is that it is a randomised, double‐blind, placebo‐controlled trial performed by family pediatricians, which is expected to increase its generalizability as compared to trials performed in tertiary care centers. The second strength is that the ARR of vomiting attributable to ginger is clinically relevant, 29 ranging from −15% under worst‐case scenario ITT to −20% under equal‐case scenario ITT. Of course, the 95% CI of the NNT is wider under the worst‐case scenario but even under this extreme and unlikely scenario, ginger preserves a clinically relevant mean effect size. Moreover, ginger is cheap and this increases its attractiveness for the treatment of AGE‐associated vomiting in children.
The present study has nonetheless some limitations. First, we did not collect any data on AGE‐associated nausea. Second, mostly for ethical reasons, we did not study children with severe dehydration. Third, we tested only a specific preparation of ginger at a fixed dose. Studies using different doses of standardized extracts are needed to determine the best preparation and dose of ginger for children with AGE.
As the primary outcome is concerned, we found that ginger is effective at preventing the occurrence of at least one episode of vomiting. Our finding that ginger is effective at improving vomiting in children with AGE is in line with studies performed in pregnant women and adults receiving chemotherapy. 38 , 39 Additional insights on the effect of ginger on pediatric AGE can be obtained from the analysis of the secondary and tertiary outcomes, although they must be taken as exploratory. Most importantly, the administration of ginger was associated with a higher intake of ORS, and with a reduction in the number of school days lost by the children.
Besides its general anti‐inflammatory effect 40 ginger contains volatile phenolic compounds such as gingerols and shogaol, that may reduce vomiting by different mechanisms. 41 In animal models, 6‐, 8‐, and 10‐gingerols, and 6‐shogaol are active on M3 receptors, 5‐HT3 receptors (guinea pigs), and 5‐HT4 receptors (rats). Gingerols and shogaol may exert their anti‐emetic effect by acting on the 5‐HT3 receptor ion‐channel complex, possibly binding to a modulatory site distinct from the serotonin binding site. This may produce indirect effects on the signal cascade behind the 5‐HT3 receptor channel complex through substance P and muscarinic receptors. 41 More recently, it was shown that the antiemetic effect of ginger may be partly dependent upon its modulating effect on the vagal nociceptive receptors of the gastrointestinal tract. 42
In conclusion, we found that ginger is effective at reducing vomiting in children with AGE. Further clinical trials are warranted to confirm our findings, to define the most effective dose of ginger, and to test whether ginger could be effective at improving vomiting of different etiologies in childhood.
AUTHORSHIP
Guarantor of the article: None.
Author contributions: RN, GC and RBC designed the study, coordinated the research team and reviewed the manuscript; RN, GB and RBC drafted the manuscript and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; RN, GC, MM, PF, GDM and MR cared for the children; RN and GB performed statistical analysis. All authors had access to the study data, reviewed and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGEMENTS
We thank the children and their families for their enthusiastic participation to the study. We also thank the family pediatricians, the nurses, and the staff members for their support during the study. We would dedicate this paper to the memory of our great colleague Dr Fabio Albano who recently passed away.
Declaration of personal interests: None.
Nocerino R, Cecere G, Micillo M, et al. Efficacy of ginger as antiemetic in children with acute gastroenteritis: a randomised controlled trial. Aliment Pharmacol Ther. 2021;54:24–31. 10.1111/apt.16404
Rita Nocerino and Gaetano Cecere contributed equally to the study.
The Handling Editor for this article was Professor Peter Gibson, and it was accepted for publication after full peer‐review.
Funding information
The study was supported by Intramural Grants from the Department of Translational Medical Science of the University of Naples Federico II. Ginger and placebo were provided by Budetta Farma Srl., Naples, Italy. Budetta Farma had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation and review of the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Parashette KR, Croffie J. Vomiting. Pediatr Rev. 2013;34:307‐319; quiz 320 [DOI] [PubMed] [Google Scholar]
- 2. Marchetti F, Bonati M, Maestro A, et al. Oral ondansetron versus domperidone for acute gastroenteritis in pediatric emergency departments: multicenter double blind randomized controlled trial. PLoS One. 2016;11:e0165441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter B, Fedorowicz Z. Antiemetic treatment for acute gastroenteritis in children: an updated Cochrane systematic review with meta‐analysis and mixed treatment comparison in a Bayesian framework. BMJ Open. 2012;2:e000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canziani BC, Uestuener P, Fossali EF, et al. Clinical practice: nausea and vomiting in acute gastroenteritis: physiopathology and management. Eur J Pediatr. 2018;177:1‐5. [DOI] [PubMed] [Google Scholar]
- 5. Zanon D, Gallelli L, Rovere F, et al. Off‐label prescribing patterns of antiemetics in children: a multicenter study in Italy. Eur J Pediatr. 2013;172:361‐367. [DOI] [PubMed] [Google Scholar]
- 6. Romano C, Dipasquale V, Scarpignato C. Antiemetic drug use in children: what the clinician needs to know. J Pediatr Gastroenterol Nutr. 2019;68:466‐471. [DOI] [PubMed] [Google Scholar]
- 7. Marzuillo P, Vecchione E, D’Anna C, Tipo V. Ondansetron as the first approach in the management of the patients with acute gastroenteritis visiting the pediatric emergency department: a single‐center experience. Turk J Gastroenterol. 2016;27:475. [DOI] [PubMed] [Google Scholar]
- 8. Li H, Liu Y, Luo D, et al. Ginger for health care: an overview of systematic reviews. Complement Ther Med. 2019;45:114‐123. [DOI] [PubMed] [Google Scholar]
- 9. Lete I, Allue J. The effectiveness of ginger in the prevention of nausea and vomiting during pregnancy and chemotherapy. Integr Med Insights. 2016;11:11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giacosa A, Morazzoni P, Bombardelli E, Riva A, Bianchi Porro G, Rondanelli M. Can nausea and vomiting be treated with ginger extract. Eur Rev Med Pharmacol Sci. 2015;19:1291‐1296. [PubMed] [Google Scholar]
- 11. Ryan JL. Treatment of chemotherapy‐induced nausea in cancer patients. Eur Oncol. 2010;6:14‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrstedt J, Dombernowsky P. Anti‐emetic therapy in cancer chemotherapy: current status. Basic Clin Pharmacol Toxicol. 2007;101:143‐150. [DOI] [PubMed] [Google Scholar]
- 13. Ansari M, Porouhan P, Mohammadianpanah M, et al. Efficacy of ginger in control of chemotherapy induced nausea and vomiting in breast cancer patients receiving doxorubicin‐based chemotherapy. Asian Pac J Cancer Prev. 2016;17:3877‐3880. [PubMed] [Google Scholar]
- 14. Arslan M, Ozdemir L. Oral intake of ginger for chemotherapy‐induced nausea and vomiting among women with breast cancer. Clin J Oncol Nurs. 2015;19:E92‐E97. [DOI] [PubMed] [Google Scholar]
- 15. Fahimi F, Khodadad K, Amini S, Naghibi F, Salamzadeh J, Baniasadi S. Evaluating the effect of zingiber officinalis on nausea and vomiting in patients receiving Cisplatin based regimens. Iran J Pharm Res. 2011;10:379‐384. [PMC free article] [PubMed] [Google Scholar]
- 16. Manusirivithaya S, Sripramote M, Tangjitgamol S, et al. Antiemetic effect of ginger in gynecologic oncology patients receiving cisplatin. Int J Gynecol Cancer. 2004;14:1063‐1069. [DOI] [PubMed] [Google Scholar]
- 17. Marx WM, Teleni L, McCarthy AL, et al. Ginger (Zingiber officinale) and chemotherapy‐induced nausea and vomiting: a systematic literature review. Nutr Rev. 2013;71:245‐254. [DOI] [PubMed] [Google Scholar]
- 18. Montazeri AS, Raei M, Ghanbari A, Dadgari A, Montazeri AS, Hamidzadeh A. Effect of herbal therapy to intensity chemotherapy‐induced nausea and vomiting in cancer patients. Iran Red Crescent Med J. 2013;15:101‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panahi Y, Saadat A, Sahebkar A, Hashemian F, Taghikhani M, Abolhasani E. Effect of ginger on acute and delayed chemotherapy‐induced nausea and vomiting: a pilot, randomized, open‐label clinical trial. Integr Cancer Ther. 2012;11:204‐211. [DOI] [PubMed] [Google Scholar]
- 20. Pillai AK, Sharma KK, Gupta YK, Bakhshi S. Anti‐emetic effect of ginger powder versus placebo as an add‐on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatr Blood Cancer. 2011;56:234‐238. [DOI] [PubMed] [Google Scholar]
- 21. Ryan JL, Heckler CE, Roscoe JA, et al. Ginger (Zingiber officinale) reduces acute chemotherapy‐induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer. 2012;20:1479‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanaati F, Najafi S, Kashaninia Z, Sadeghi M. Effect of ginger and chamomile on nausea and vomiting caused by chemotherapy in Iranian women with breast cancer. Asian Pac J Cancer Prev. 2016;17:4125‐4129. [PubMed] [Google Scholar]
- 23. Thamlikitkul L, Srimuninnimit V, Akewanlop C, et al. Efficacy of ginger for prophylaxis of chemotherapy‐induced nausea and vomiting in breast cancer patients receiving adriamycin‐cyclophosphamide regimen: a randomized, double‐blind, placebo‐controlled, crossover study. Support Care Cancer. 2017;25:459‐464. [DOI] [PubMed] [Google Scholar]
- 24. Zick SM, Ruffin MT, Lee J, et al. Phase II trial of encapsulated ginger as a treatment for chemotherapy‐induced nausea and vomiting. Support Care Cancer. 2009;17:563‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. 2000;84:367‐371. [DOI] [PubMed] [Google Scholar]
- 26. Lee J, Oh H. Ginger as an antiemetic modality for chemotherapy‐induced nausea and vomiting: a systematic review and meta‐analysis. Oncol Nurs Forum. 2013;40:163‐170. [DOI] [PubMed] [Google Scholar]
- 27. Nikkhah Bodagh M, Maleki I, Hekmatdoost A. Ginger in gastrointestinal disorders: a systematic review of clinical trials. Food Sci Nutr. 2019;7:96‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marx W, Kiss N, Isenring L. Is ginger beneficial for nausea and vomiting? An update of the literature. Curr Opin Support Palliat Care. 2015;9:189‐195. [DOI] [PubMed] [Google Scholar]
- 29. Freedman SB, Adler M, Seshadri R, Powell EC. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354:1698‐1705. [DOI] [PubMed] [Google Scholar]
- 30. Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 31. Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence‐based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132‐152. [DOI] [PubMed] [Google Scholar]
- 32. Ryan P. RALLOC: Stata module to design randomized controlled trials. Statistical Software Components. 1997. https://ideasrepecorg/c/boc/bocode/s319901html
- 33. WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards: Length Height‐For‐Age, Weight‐For‐Age, Weight‐For‐Length, Weight‐For‐Height And Body Mass Index‐For‐Age: Methods And Development. Geneva: World Health Organization; 2006. [Google Scholar]
- 34. Hardin JW, Hilbe JM. Generalized Linear Models and Extensions, 3rd ed. College Station, TX: Stata Press; 2018. [Google Scholar]
- 35. Bender R. Calculating confidence intervals for the number needed to treat. Control Clin Trials. 2001;22:102‐110. [DOI] [PubMed] [Google Scholar]
- 36. Berni Canani R, Di Costanzo M, Bedogni G, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3‐year randomized controlled trial. J Allergy Clin Immunol. 2017;139:1906‐1913.e4 [DOI] [PubMed] [Google Scholar]
- 37. Rabe‐Hesketh S. Multilevel and Longitudinal Modeling Using Stata. Volume II: Categorical Responses, Counts, and Survival. 3rd ed. College Station, TX: Stata Press; 2012. [Google Scholar]
- 38. Marx W, Ried K, McCarthy AL, et al. Ginger‐mechanism of action in chemotherapy‐induced nausea and vomiting: a review. Crit Rev Food Sci Nutr. 2017;57:141‐146. [DOI] [PubMed] [Google Scholar]
- 39. Sharifzadeh F, Kashanian M, Koohpayehzadeh J, Rezaian F, Sheikhansari N, Eshraghi N. A comparison between the effects of ginger, pyridoxine (vitamin B6) and placebo for the treatment of the first trimester nausea and vomiting of pregnancy (NVP). J Matern Fetal Neonatal Med. 2018;31:2509‐2514. [DOI] [PubMed] [Google Scholar]
- 40. Semwal RB, Semwal DK, Combrinck S, Viljoen AM. Gingerols and shogaols: important nutraceutical principles from ginger. Phytochemistry. 2015;117:554‐568. [DOI] [PubMed] [Google Scholar]
- 41. Pertz HH, Lehmann J, Roth‐Ehrang R, Elz S. Effects of ginger constituents on the gastrointestinal tract: role of cholinergic M3 and serotonergic 5‐HT3 and 5‐HT4 receptors. Planta Med. 2011;77:973‐978. [DOI] [PubMed] [Google Scholar]
- 42. Huang Y, Patil MJ, Yu M, et al. Effects of ginger constituent 6‐shogaol on gastroesophageal vagal afferent C‐fibers. Neurogastroenterol Motil. 2019;31:e13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


