Table 1.
Optimisation studies.
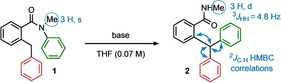
|
Entry[a] |
Base (equiv) |
T [°C][b] |
t [h] |
Yield [%][c] |
|---|---|---|---|---|
|
1 |
NaHMDS (2.0) |
20 |
16 |
<5 |
|
2 |
NaHMDS (2.0) |
60 |
16 |
49 |
|
3 |
NaHMDS (2.0) |
100 |
1 |
73 |
|
4 |
LiHMDS (2.0) |
100 |
1 |
62 |
|
5 |
KHMDS (2.0) |
100 |
1 |
81 (67) |
|
6 |
KHMDS (1.1) |
100 |
1 |
70 |
|
7 |
KHMDS (2.0) |
120 |
1 |
69 |
[a] Reactions performed on a 0.1 mmol scale. KHMDS (1.0 m in THF)=potassium bis(trimethylsilyl)amide, NaHMDS (1.0 m in THF)=sodium bis(trimethylsilyl)amide, LiHMDS (1.0 m in THF)=lithium bis(trimethylsilyl)amide. [b] Reactions requiring 60 °C and below were heated conventionally. Reactions requiring heating above 60 °C were performed under microwave irradiation. [c] Yield determined by 1H NMR using 1,3,5‐trimethoxybenzene as an internal standard. Isolated yield in parentheses (0.2 mmol scale reaction).
