Fig. 3.
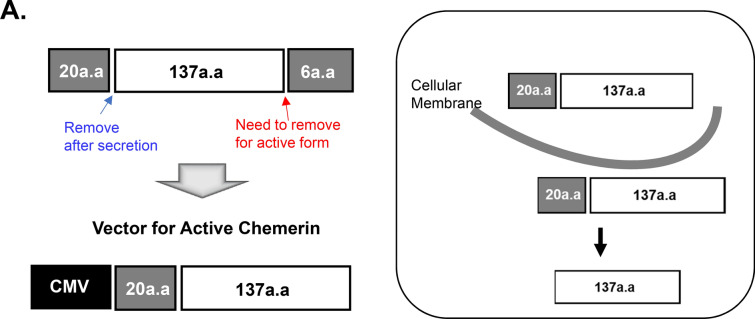
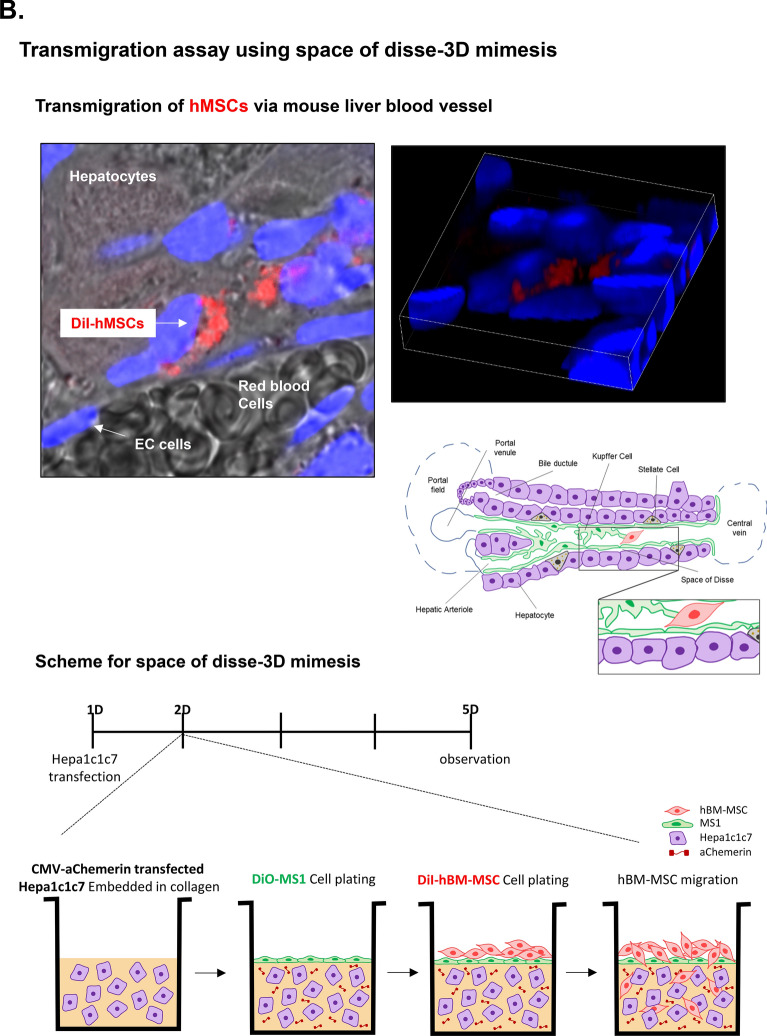
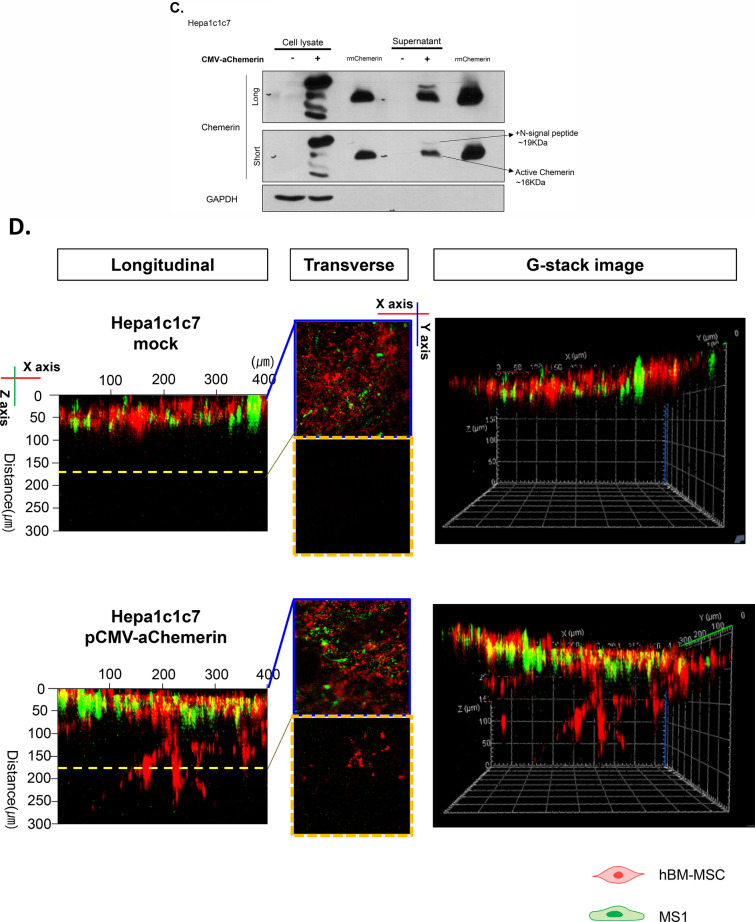
Construction of active chemerin vector. A Scheme showing the active chemerin vector. Twenty amino acids at N-terminus represent the signaling peptide that assists secretion of chemerin from hMSCs. The last six amino acids at C-terminus are enzymatically cleaved away leaving active chemerin. B Scheme for 3D mimesis of the space of Disse. The upper panel shows the possibility of transendothelial migration of hBM-MSCs in the space of Disse. To observe the possibility of ex vivo migration, a similar environment was constructed using Hepa-1c1c7 (hepatocytes), MS-1 (endothelial cells), and hBM-MSCs. C Evaluation of the vector in Hepa-1c1c7. Levels of the vector-encoded protein in the lysate and media of Hepa-1c1c7 cells transfected with the vector were detected using western blotting. The cell lysate shows different-sized chemerin and the supernatant shows inactive and active chemerin. Recombinant mouse chemerin (rmChemerin) was loaded as the positive control. D Transendothelial migration of hBM-MSCs. Penetration of hBM-MSCs through MS-1 cells into collagen matrix containing Hepa-1c1c7 cells transfected with active chemerin vector. MS-1 was labeled with DiO (green) and hBM-MSC was labeled with DiI (red). Migration in the vertical side of the collagen matrix was observed using the Z stack of the confocal microscope. The migration distances of hBM-MSCs were indicated in the scale of 50 µM. The data shown were reproducible results from two independent experiments