Scheme 6.
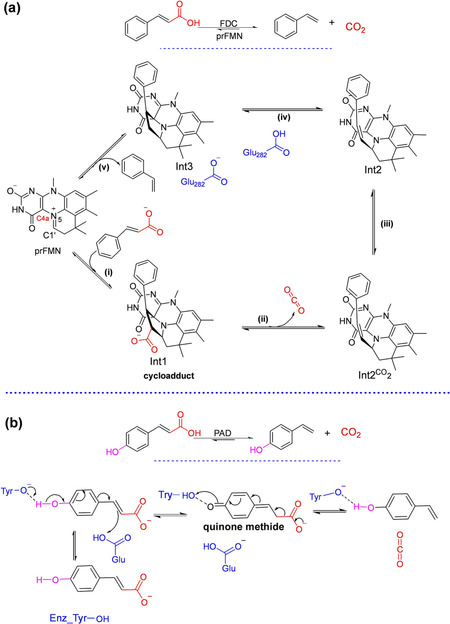
Mechanism of FDC‐catalyzed (de)carboxylation of phenylacrylic acids (e. g., cinnamic acid) vs PAD‐mediated (de)carboxylation of phenolic analogues (e. g., p‐coumaric acid). (a) Mechanism for decarboxylation of cinnamic acid by covalent catalysis using the prFMN iminium involving an initial 1,3‐dipolar cycloaddition between the dipolarophile of the substrate and the azomethine ylide‐like species of prFMNiminium resulting in a cycloadduct species Int1. The Glu282 side chain mediates the protonation step to form Int3.[ 70 , 71 ] The decarboxylation product styrene is released through a cycloelimination process. (i–v)=1,3‐dipolar cycloaddition, decarboxylation, CO2 to Glu 282 exchange, protonation and cycloelimination, respectively. (b) A general acid‐base mechanism employed by phenolic acid decarboxylases (PADs) and enabled by an essential phenolic moiety.[ 52 , 72 ]
