Figure 5.
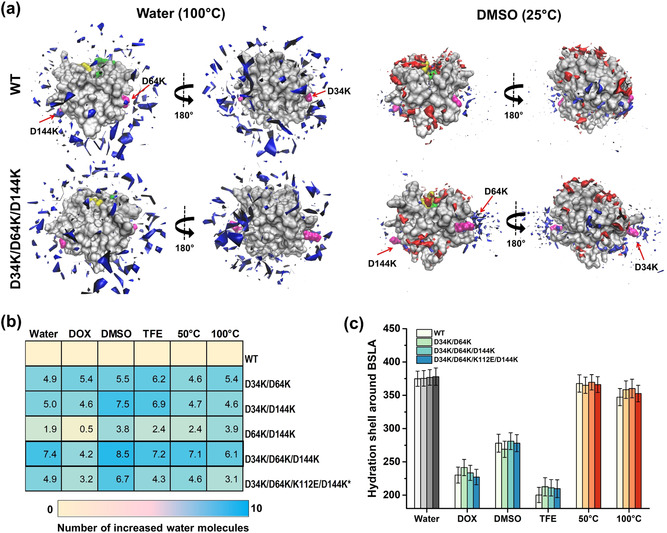
Solvation phenomenon of BSLA variants in cosolvents and at different temperatures. a) Spatial distribution of water and/or OS molecules at the molecular surface of the BSLA WT and D34K/D64K/D144K in water (100 °C) and DMSO (25 °C). The BSLA surface is shown in grey, Ser77, Asp133, His156 (the catalytic triad) in green, and Ile12, Met78 (oxyanion hole) in yellow; the OS molecules in red, the water molecules in blue. The substitutions are shown in magenta and indicated by the red arrow. The 180‐degree rotation is offered to give a complete view of BSLA. The contours are shown with isovalue 10 for water and isovalue 14 for DMSO molecules. b) Heatmap indicating the number of water molecules around the substituted sites averaged over the last 40 ns of MD trajectories. The substituted sites include positions 34, 64, 112, and 144. Asterisks: only two unfavorable salt bridges were removed in D34K/D64K/K112E/D144K. c) Hydration shell around BSLA variants averaged over the last 40 ns of MD trajectories. The hydration shell is defined as water molecules whose oxygen atom is localized at a distance ≤3.5 Å from any non‐hydrogen atom of the protein.[ 9b , 51 ] The number of water molecules is defined as hydration level. [51] For better comparison, grey, blue, and red colors were used to indicate water, OSs, and high temperatures, respectively.
