Abstract
Background and Aims
Previous recommendations suggested living donor liver transplantation (LDLT) should not be considered for patients with Model for End‐Stage Liver Disease (MELD) > 25 and hepatorenal syndrome (HRS).
Approach and Results
Patients who were listed with MELD > 25 from 2008 to 2017 were analyzed with intention‐to‐treat (ITT) basis retrospectively. Patients who had a potential live donor were analyzed as ITT‐LDLT, whereas those who had none belonged to ITT‐deceased donor liver transplantation (DDLT) group. ITT‐overall survival (OS) was analyzed from the time of listing. Three hundred twenty‐five patients were listed (ITT‐LDLT n = 212, ITT‐DDLT n = 113). The risk of delist/death was lower in the ITT‐LDLT group (43.4% vs. 19.8%, P < 0.001), whereas the transplant rate was higher in the ITT‐LDLT group (78.3% vs. 52.2%, P < 0.001). The 5‐year ITT‐OS was superior in the ITT‐LDLT group (72.6% vs. 49.5%, P < 0.001) for patients with MELD > 25 and patients with both MELD > 25 and HRS (56% vs. 33.8%, P < 0.001). Waitlist mortality was the highest early after listing, and the distinct alteration of slope at survival curve showed that the benefits of ITT‐LDLT occurred within the first month after listing. Perioperative outcomes and 5‐year patient survival were comparable for patients with MELD > 25 (88% vs. 85.4%, P = 0.279) and patients with both MELD > 25 and HRS (77% vs. 76.4%, P = 0.701) after LDLT and DDLT, respectively. The LDLT group has a higher rate of renal recovery by 1 month (77.4% vs. 59.1%, P = 0.003) and 3 months (86.1% vs, 74.5%, P = 0.029), whereas the long‐term estimated glomerular filtration rate (eGFR) was similar between the 2 groups. ITT‐LDLT reduced the hazard of mortality (hazard ratio = 0.387‐0.552) across all MELD strata.
Conclusions
The ITT‐LDLT reduced waitlist mortality and allowed an earlier access to transplant. LDLT in patients with high MELD/HRS was feasible, and they had similar perioperative outcomes and better renal recovery, whereas the long‐term survival and eGFR were comparable with DDLT. LDLT should be considered for patients with high MELD/HRS, and the application of LDLT should not be restricted with a MELD cutoff.
Abbreviations
- AKI
acute kidney injury
- BMI
body mass index
- DDLT
deceased donor liver transplantation
- eGFR
estimated glomerular filtration rate
- GRWR
graft‐to‐recipient weight ratio
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- HRS
hepatorenal syndrome
- ICU
intensive care unit
- ITT
intention to treat
- LDLT
living donor liver transplantation
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- MHV
middle hepatic vein
- MOF
multiorgan failure
- OS
overall survival
- RRT
renal replacement therapy
The utilization of living donor liver transplantation (LDLT) for patients with high Model for End‐Stage Liver Disease (MELD) scores is controversial.( 1 ) Although there is no consensus for a universal definition of high MELD in the literature, it is usually arbitrarily defined as MELD > 25.( 1 ) In 2002, the New York State Committee on Quality Improvement in Living Liver Donation recommended that LDLT should be excluded for candidates with MELD score > 25 and/or need for renal replacement therapy (RRT), although such a recommendation might change as more data become available.( 2 ) This was based on early reports that LDLT was associated with an unacceptably high risk of mortality and graft loss in this group of patients.( 3 , 4 ) The limited application of LDLT for patients with high MELD in the United States was reflected by the fact that only 54 LDLTs were performed for patients with MELD > 25 from 2002 to 2008.( 5 ) In recent decades, the outcomes of LDLT for patients with high MELD have improved,( 6 , 7 , 8 , 9 ) and centers have explored whether the use of LDLT for patients with high MELD can be justified and beneficial.( 5 , 10 )
LDLT offers an opportunity to save lives for these patients who are critically ill who would otherwise have a dismal chance of survival without transplant. This is especially important when timely access to deceased donor livers is unlikely. On the other hand, the benefits of recipients should be weighed against the risk and cost of living donation, which includes donor coercion under an emergent and expedited workup process and possibly inferior transplant outcomes.( 11 ) In the literature, all reports that compared LDLT and deceased donor liver transplantation (DDLT) for patients with high MELD and hepatorenal syndrome (HRS) only analyzed outcomes and survival after transplant, without any analysis of the effect of type of transplant on waitlist mortality.( 3 , 4 , 5 , 6 , 16 ) Furthermore, it is unclear whether the use of expedited living donor workup will reduce waitlist mortality and what the impact will be on perioperative donor and recipient outcomes.
In this study we aimed to investigate the role and benefits of LDLT for patients with high MELD and HRS on an intention‐to‐treat (ITT) basis and to evaluate the short‐term renal recovery and long‐term renal outcomes in patients with HRS. Also, the outcomes of living donation under an expedited workup process would also be evaluated.
Patients and Methods
This was a retrospective study from 2008 to 2017 in a university center. Data were retrieved from a prospectively collected database. All patients who were accepted for transplant with an actual MELD score > 25 at the time of listing were included. Patients who had retransplantation, dual living donors, and domino transplant were excluded. The study was approved by the institutional review board (UW 19‐314) and met the requirement of the Declaration of Helsinki. The need for informed consent was waived. In this study, recipients were analyzed on an ITT basis. Patients were classified into the ITT‐LDLT group if they had a potential candidate who commenced donor evaluation. If during the donor evaluation the potential candidate was found unsuitable, the reason for ineligibility would be recorded. Alternatively, patients were classified as ITT‐DDLT group if they had no living donor available or refused LDLT at the very beginning. No organ donors were obtained from executed prisoners or other institutionalized persons.
Waitlist Management and Outcomes
All candidates were evaluated by a multidisciplinary team including transplant surgeons, hepatologists, cardiologists, respiratory physicians, anesthetists, clinical psychologists, and nurse specialists. The option of LDLT was explained to all candidates. HRS was defined as a renal failure that occurred in patients with end‐stage liver disease after ruling out other causes of renal failure according to previous consensus. The diagnosis of HRS was defined according to the International Club of Ascites (ICA) criteria for acute kidney injury (AKI) in patients with cirrhosis. HRS‐AKI was defined as ICA‐AKI ≥ stage II (AKI stage II: increase in serum creatinine >2‐3–fold from baseline), no response to diuretic withdrawal, and plasma volume expansion with albumin 1 g per kg of body weight and, in the absence of shock, nephrotoxic drug and structural kidney injury.( 17 ) Treatment of HRS consisted of intravenous terlipressin (Ferring Pharmaceuticals, Switzerland) given at 0.5‐1 mg every 4‐6 hours together with intravenous albumin at 40 g per day.( 18 , 19 ) RRT would be initiated in patients with HRS who developed persistent hyperkalemia, acidosis, and oliguria. All patients were prioritized according to MELD score. The presence of living donors would not alter the waitlist priority of the recipients, and they would proceed to DDLT if deceased organs were available. Recipients who required ventilatory, cardiovascular support and RRT would be managed in the intensive care unit (ICU). Candidates who had high MELD, were critically ill, or required organ support were not contraindicated to LDLT. The contraindications for transplant were the same for LDLT and DDLT and included overwhelming sepsis, cerebral edema, multiorgan failure (MOF), and hepatocellular carcinoma (HCC) progression. The causes for delisting or death while on waitlist were recorded. In addition, the medical records of all patients who did not reach transplant were reviewed and appraised whether the recipient might be salvageable by the presence of a living donor.
Donor Evaluation
All living donations were voluntary. Donor and recipient evaluations were conducted simultaneously. Donor evaluations were conducted as in‐patient if recipients were critically ill. The pairs were interviewed by transplant surgeons, transplant hepatologists, and nurse specialists. The process of donor evaluations was expedited but the workup protocols were equivalent to elective setting. Donors should have no acute or chronic medical condition(s) that would increase operative risk. The initial assessment consisted of a detailed medical history and measurement of weight, height, body mass index (BMI), blood pressure, and urinalysis. Baseline blood tests included complete blood count; liver function test; renal function test; fasting glucose and lipid; serologies for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus; and blood typing. Donor candidates with BMI > 30 kg/m2 were considered ineligible for living donation. For those with BMI > 27‐30 kg/m2, a percutaneous liver biopsy would be performed, and the presence of macrovesicular steatosis >10% would be unfit for living donation. Additional investigations were performed based on individual assessments, including mammography for female donors >35 years of age; echocardiogram, treadmill, and carotid duplex for those with risk factors for cardiovascular and cerebrovascular disease; and colonoscopy and esophagogastroscopy for those with anemia. From 2012, ABO‐incompatible LDLT became available for pairs who had incompatible blood groups.
As part of the standard evaluation process, donor candidates were also interviewed by a nontransplant surgeon who was not involved in recipient care. In addition, all donor candidates were assessed by clinical psychologists for any psychological contraindication for living donation. Contrast computed tomography with liver volumetry was performed, and the graft weight was estimated by dividing the liver volume by 1.19, which was then used to calculate the predicted graft‐to‐recipient weight ratio (GRWR). Although a predicted GRWR > 0.8% is preferable, GRWR ≥ 0.6%‐0.8% can be allowed in the following situations: (1) absence of alternative living donor; (2) primary transplant; (3) recipient has no >2 organ failures; and (4) both donors and recipients accept a higher perioperative morbidity and mortality rate.( 20 ) Approval must be obtained from an independent statutory human organ transplant board for LDLT between persons who were not genetically related or a couple whose marriage has subsisted for <3 years.( 21 )
Transplant Operation
Operation was standardized as described( 22 ) (Supporting Information).
Postoperative and Immunosuppression Protocol
Postoperative and immunosuppression protocol was identical after LDLT and DDLT (Supporting Information).
Postoperative Protocol for Patients With HRS
Terlipressin was stopped after transplant in patients with HRS. Estimated glomerular filtration rate (eGFR) was calculated and monitored based on the Modification of Diet in Renal Disease equation at every clinic follow‐up.( 23 ) Recovery of renal function was defined as recovery of eGFR ≥ 60 mL/min/1.73 m2 after AKI.
Statistical Analysis
Continuous parameters were summarized by median and range unless otherwise specified. Categorical variables were expressed by frequency and percentage. Comparison between groups was performed using Pearson’s chi‐squared test for categorical variables or the Mann‐Whitney U test for continuous variables. ITT‐overall survival (OS) was measured from the time of listing to death from any cause. Patient survival after transplant was determined from the time of transplant to death from any cause. Graft survival was measured from the time of transplant to recipient demise, being listed for retransplantation, or retransplantation. Survival was analyzed using the Kaplan‐Meier method and compared using the log‐rank test. A sensitivity analysis was carried out using the Breslow (generalized Wilcoxon) test for ITT‐OS.( 24 ) Cox regression analysis was used to define variables that predicted ITT‐OS and survival after transplant. Univariate analysis was performed using factors related to recipients’ and donors’ demographics, graft size, disease etiology, operative details, and postoperative events. The Cox proportional hazard assumption was checked using Schoenfeld’s global test.( 25 , 26 ) Significant factors from univariate analysis (P < 0.1) were entered for multivariable analysis. Statistical significance was defined as P value <0.05, and all tests were performed two‐tailed. All calculations were done using SPSS25.0 and GraphPad Prism8.0.
Results
During the study period, 325 patients were listed with a MELD score >25. Two hundred and twelve (65.2%) patients were in the ITT‐LDLT group, and 113 (34.8%) patients did not have a living donor (ITT‐DDLT). The median follow‐up time for the whole cohort was 43.3 (0‐136) months: 51.7 (0‐136) months for the ITT‐LDLT group and 18.1 (0‐135.3) months for the ITT‐DDLT group (P = 0.01). Figure 1 shows the waitlist outcomes of all patients. In the ITT‐DDLT group, 59 (52.2%) underwent transplant, 49 (43.4%) patients died on waitlist or were delisted, 4 (3.5%) recovered, and 1 (0.9%) remained alive on waitlist. On the other hand, in the ITT‐LDLT group, 166 (78.3%) underwent transplant, 42 (19.8%) patients died on waitlist or were delisted, and 4 (1.9%) recovered. The chance of getting a transplant was much higher in the ITT‐LDLT group (78.3% vs. 52.2%, P < 0.001) (Fig. 1). Among the 49 patients who were delisted or died on waitlist in the ITT‐DDLT group, 19/49 (38.8%) were considered irreversible, i.e., too sick for transplant. The reasons for delisting or death were MOF in 7 patients, cerebral edema in 6, cardiac event in 3, tumor progression in 2, and overwhelming sepsis in 1. In the ITT‐LDLT group, 9/42 (21.4%) patients were considered irreversible. The reasons were MOF in 7 and cerebral edema in 2.
FIG. 1.
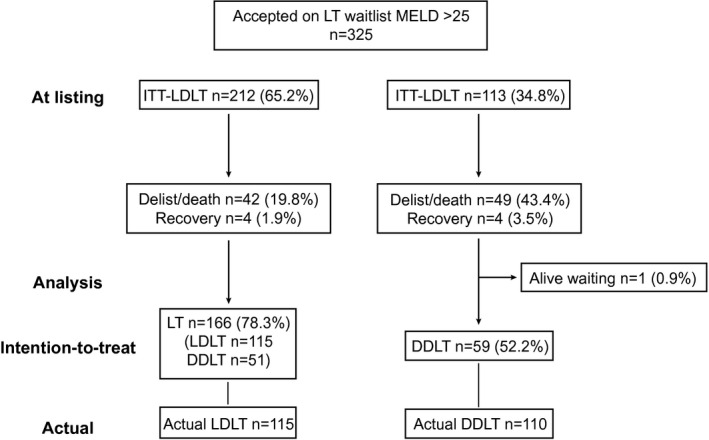
Flow chart of all patients who were listed with MELD > 25.
In the ITT‐LDLT group, 20 donor candidates were ineligible for living donation (ABO incompatible before year 2012, n = 5; BMI > 30/cardiovascular disease, n = 4; hepatitis B virus/hepatitis C virus carrier, n = 2; donor anatomical contraindication, n = 2; donor psychological/psychiatric condition, n = 4; and other medical diseases, n = 3). As for their respective recipients, 5 eventually underwent DDLT.
Clinical Characteristics at Listing and Waitlist Outcomes
There was no difference in baseline demographics, including recipient age, BMI, hepatitis status, and MELD at listing (Table 1). There were more male patients in the ITT‐DDLT group (85.8% vs. 74.1%, P = 0.014). Very high MELD, defined as MELD > 35, was seen in 46% and 42.9% of the ITT‐DDLT and ITT‐LDLT groups, respectively (P = 0.593). There were more patients with HRS in the ITT‐DDLT group (65.5% vs. 53.8%, P = 0.034). The median eGFR at listing was similar in both groups (42.8 vs. 48.2 mL/min/1.73 m2, P = 0.089) and around one third of the patients in both groups had eGFR < 30 mL/min/1.73 m2. Subgroup analysis of MELD > 30 and MELD > 35 at listing showed similar findings (Supporting Table S1).
TABLE 1.
Clinical Characteristics of ITT‐LDLT and ITT‐DDLT Groups at Time of Listing
| ITT‐DDLT Group (n = 113) | ITT‐LDLT Group (n = 212) | P Value | |
|---|---|---|---|
| Age (years) | 55.5 (34.6‐72.5) | 56.7 (24.9‐75.8) | 0.684 |
| Recipient sex male (n, %) | 97 (85.8) | 157 (74.1) | 0.014 |
| Recipient BMI (kg/m2) | 24 (17‐43.9) | 24.5 (16.4‐42.9) | 0.913 |
| Hepatitis B virus (n, %) | 89 (78.8) | 164 (77.4) | 0.772 |
| Hepatitis C virus (n, %) | 3 (2.7) | 4 (1.9) | 0.958 |
| Disease indication (n, %) | 0.024 | ||
| Acute liver failure | 10 (8.8) | 20 (9.4) | |
| Acute on chronic liver failure | 38 (33.6) | 98 (46.2) | |
| Decompensated cirrhosis | 53 (46.9) | 73 (34.4) | |
| With concomitant HCC | 9 (8) | 7 (3.3) | |
| Others | 3 (2.7) | 14 (6.6) | |
| At listing | |||
| Bilirubin (μmol/L) | 443 (59‐1,044) | 434.5 (29‐949) | 0.508 |
| INR | 2.7 (1.5‐8) | 3 (1.4‐10) | 0.156 |
| Creatinine (μmol/L) | 155 (74‐766) | 136 (62‐1,022) | 0.055 |
| eGFR (mL/min/1.73 m2)* | 42.8 (5.8‐138) | 48.2 (4.3‐136.5) | 0.089 |
| <30 | 41 (36.3) | 67 (31.6) | |
| 30‐60 | 37 (32.7) | 54 (25.5) | |
| >60 | 35 (31) | 91 (42.9) | |
| MELD at listing | 34.2 (25.2‐51.2) | 34.1 (25.2‐55) | 0.840 |
| MELD > 35 at listing (n, %) | 52 (46) | 91 (42.9) | 0.593 |
| HRS (n, %) | 74 (65.5) | 113 (53.8) | 0.034 |
Note: Data are presented as n (%) or median (range).
eGFR was based on the Modification of Diet in Renal Disease equation.
ITT Survival of MELD > 25 and HRS
Figure 2A,B shows the long‐term ITT‐OS of patients who had MELD > 25 and both MELD > 25 and HRS. For those who had a listing MELD > 25, the 1‐year, 3‐year, and 5‐year ITT‐OS were significantly better in the ITT‐LDLT group (76.4% vs. 52.2%, 74% vs. 49.5%, and 72.6% vs. 49.5%, respectively; P < 0.001). The ITT‐OS of patients with MELD > 25 and HRS at 1, 3, and 5 years were 61.9% vs. 35.1%, 57.4% vs. 33.8%, and 56% vs. 33.8% in the ITT‐LDLT HRS and ITT‐DDLT HRS groups, respectively (P < 0.001). Figure 2C showed that early ITT‐OS at 1, 4, 8, and 12 weeks after listing in patients with MELD > 25 was better in the ITT‐LDLT group (93.4% vs. 84.1%, 85.8% vs. 61.9%, 82.5% vs. 59.3%, and 82.1% vs. 58.4%; P < 0.001). Similar early survival benefit was observed in the ITT‐LDLT group for patients with MELD > 25 and HRS (88.5% vs. 78.4%, 76.1% vs. 48.6%, 70.8% vs. 43.2%, 69.9% vs. 40.5%; P < 0.001) (Fig. 2D). As most of the deaths occurred early after listing, with a distinct alteration in the slope of the survival curves at 1 month after listing, an ITT‐OS that analyzed patients who survived for >1 week (Fig. 2E), >1 month (Fig. 2F), and >3 months (Supporting Fig. S1) showed that the survival benefits of ITT‐LDLT occurred within the first month after listing.
FIG. 2.
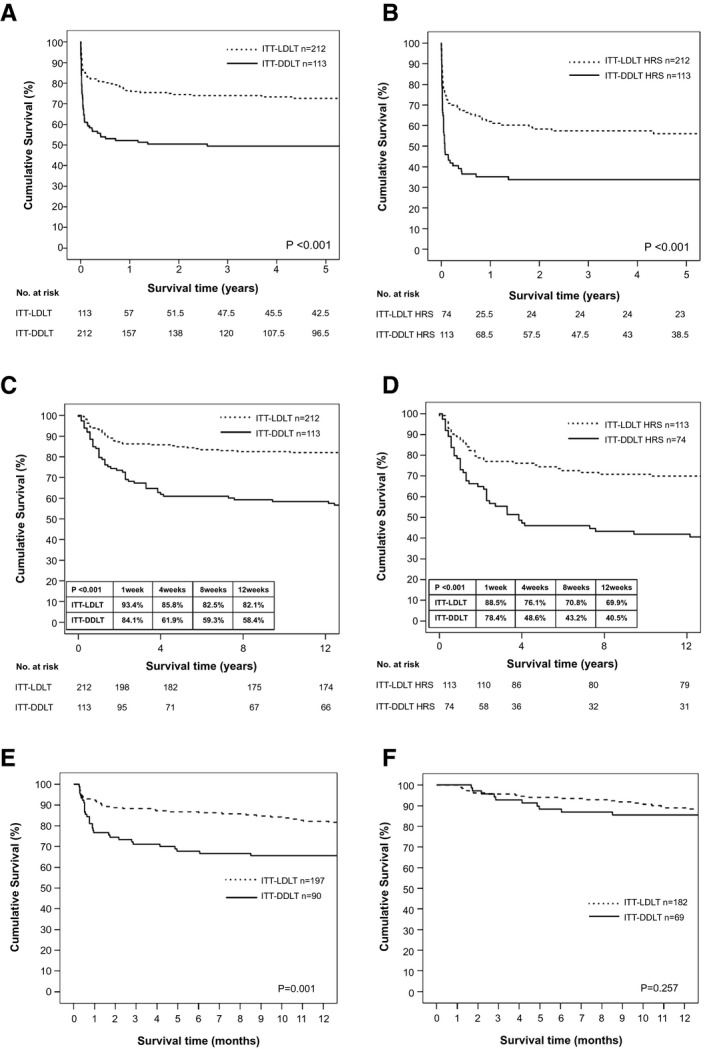
(A) ITT survival of patients with MELD > 25. (B) ITT survival of patients with MELD > 25 and HRS. (C) ITT early survival of patients with MELD > 25. (D) ITT early survival of patients with MELD > 25 and HRS. (E) ITT survival of patients who survived >1 week from listing. (F) ITT survival of patients who survived >1 month from listing.
A more distinct survival benefit was observed in those who had a listing MELD > 35; the 1‐year, 3‐year, and 5‐year survival rates were 68.1%, 64.7%, and 63.1% in the ITT‐LDLT group compared with 30.8%, 30.8%, and 30.8% in the ITT‐DDLT group, respectively (P < 0.001) (Supporting Fig. S2A). Similar survival benefits were observed among patients with HRS as well. In patients with MELD > 35 and HRS, the 1‐year, 3‐year, and 5‐year ITT‐OS was better in the ITT‐LDLT HRS group (59.4% vs. 25%, 54.5% vs. 25%, and 54.5% vs. 25%, respectively; P = 0.001) (Supporting Fig. S2B).
Sensitivity Analysis of ITT Survival
In addition to the log‐rank test for survival comparison, the Breslow test was used as a sensitivity analysis to understand whether putting more weight on early events or failure would alter our findings. With the Breslow test, the survival benefits in the ITT‐LDLT group in MELD > 25 (P < 0.001), MELD > 35 (P < 0.001), MELD > 25 and HRS (P < 0.001), and MELD > 35 and HRS (P = 0.001) remain unchanged.
Predictors of Survival From the Time of Listing
The proportionality of hazard assumption in Cox regression was confirmed using Schoenfeld’s global test with P = 0.421, i.e., absence of a significant time‐varying effect. In multivariable analysis on mortality from the time of listing, patients with higher BMI (HR, 0.912; 95% CI, 0.866‐0.96; P < 0.001) and in the ITT‐LDLT group (HR, 0.392; 95% CI, 0.273‐0.562; P < 0.001) had a lower risk of mortality, whereas a higher MELD score at listing predicted poorer survival (HR, 1.082; 95% CI, 1.052‐1.112; P < 0.001) (Table 2).
TABLE 2.
Univariate and Multivariable Analyses for Prognostic Factors Affecting Mortality From Time of Listing With ITT Analysis
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR, 95% CI | P Value | HR, 95% CI | P Value | |
| Recipient age (n = 325) | 0.984 (0.967‐1.000) | 0.051 | ||
| Recipient sex (n = 325) | 0.143 | |||
| Female (n = 71) | Ref | |||
| Male (n = 254) | 1.422 (0.888‐2.277) | |||
| Recipient BMI (n = 325) | 0.932 (0.886‐0.980) | 0.006 | 0.912 (0.866‐0.960) | <0.001 |
| Disease etiology (n = 325) | 0.578 | |||
| No HBV (n = 72) | Ref | |||
| HBV infection (n = 253) | 0.888 (0.585‐1.349) | |||
| MELD at listing (n = 325) | 1.070 (1.041‐1.100) | <0.001 | 1.082 (1.052‐1.112) | <0.001 |
| HCC (n = 325) | 0.015 | |||
| No (n = 309) | Ref | |||
| Yes (n = 16) | 2.169 (1.165‐4.038) | |||
| Transplant type (n = 325) | <0.001 | <0.001 | ||
| ITT‐DDLT (n = 113) | Ref | |||
| ITT‐LDLT (n = 212) | 0.433 (0.302‐0.620) | 0.392 (0.273‐0.562) | ||
Note: Data are presented as n (%) or median (range).
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; Ref, reference group.
Clinical Characteristics at Transplant and Perioperative Outcomes
Regarding patients who underwent DDLT and LDLT, there was no difference in age, sex, BMI, hepatitis status, disease indication, MELD at transplant, or HRS, and most patients were hospitalized or required ICU care before transplant. There were more patients who had MELD > 35 (43.5% vs. 30%, P = 0.036) in the LDLT group. The median eGFR at transplant was comparable (74.5 vs. 68.9 mL/min/1.73 m2, P = 0.693) and the duration of HRS before transplant was 7 and 6 days in the DDLT and LDLT groups, respectively (P = 0.656). The median time on the waitlist was longer in patients with DDLT (15.5 vs. 7 days P < 0.001) (Table 3). As expected, graft weight and GRWR was lower in the LDLT group, with shorter cold ischemic time and longer operative time compared with the DDLT group. The majority of patients with LDLT underwent transplantation with right‐lobe grafts (111/115, 96.5%) and most were inclusive of middle hepatic veins (MHV; 106/111, 95.5%). There was no difference between the two groups in terms of ICU stay, hospital stay, vascular and biliary complications, and hospital mortality. The overall and severe perioperative complication rates were lower in the LDLT group (Table 4). Subgroup analysis of perioperative outcomes of recipients with MELD > 30 and MELD > 35 showed comparable outcomes after LDLT and DDLT (Supporting Table S2).
TABLE 3.
Clinical Characteristics of LDLT and DDLT Groups at Time of Transplant
| Parameters | DDLT Group (n = 110) | LDLT Group (n = 115) | P Value |
|---|---|---|---|
| Recipient | |||
| Age (years) | 55.6 (24.9‐72.5) | 58.1 (25.5‐75.8) | 0.131 |
| Recipient sex male (n, %) | 87 (79.1) | 86 (74.8) | 0.443 |
| Recipient BMI (kg/m2) | 24.5 (17.5‐43.9) | 24.6 (14.4‐42.9) | 0.941 |
| Hepatitis B virus (n, %) | 85 (77.3) | 91 (79.1) | 0.736 |
| Hepatitis C virus (n, %) | 4 (3.6) | 2 (1.7) | 0.639 |
| Disease indication (n, %) | 0.455 | ||
| Acute liver failure | 5 (4.5) | 10 (8.7) | |
| Acute on chronic liver failure | 56 (50.9) | 47 (40.9) | |
| Decompensated cirrhosis | 38 (34.5) | 48 (41.7) | |
| With concomitant HCC | 5 (4.5) | 4 (3.5) | |
| Others | 6 (5.5) | 6 (5.2) | |
| At transplant | |||
| Bilirubin (μmol/L) | 429.5 (21‐1,017) | 442 (112‐1,022) | 0.523 |
| INR | 2.6 (1.2‐10.1) | 2.8 (1.2‐5.3) | 0.072 |
| Creatinine (μmol/L) | 97.5 (35‐841) | 102 (30‐512) | 0.867 |
| eGFR (mL/min/1.73 m2)* | 74.5 (4.5‐138) | 68.9 (9.5‐142.3) | 0.693 |
| <30 | 21 (19.1) | 26 (22.6) | |
| 30‐60 | 26 (23.6) | 26 (22.6) | |
| >60 | 63 (57.3) | 63 (53.8) | |
| MELD at transplant | 31.4 (9.3‐49.8) | 33 (19‐53) | 0.140 |
| MELD > 35 at transplant (n, %) | 33 (30) | 50 (43.5) | 0.036 |
| HRS (n, %) | 55 (50) | 52 (45.2) | 0.473 |
| HRS and required perioperative RRT (n, %) | 8 (7.3) | 13 (11.3) | 0.174 |
| Duration of HRS before LT (days) | 7 (1‐92) | 6 (1‐80) | 0.656 |
| Known renal impairment before LT (n, %) | 5 (4.5) | 4 (3.5) | 0.683 |
| Pretransplant status (n, %) | 0.145 | ||
| Home | 12 (10.9) | 2 (1.7) | |
| Hospitalized | 65 (59.1) | 57 (49.6) | |
| ICU | 33 (30) | 56 (48.7) | 0.360 |
| Liver failure only | 14 (42.4) | 18 (32.1) | |
| Liver failure + 1 organ failure † | 18 (54.5) | 36 (64.3) | |
| Liver failure + 2 organs failure † | 1 (3) | 2 (3.6) | |
| Waiting time (days) | 15.5 (1‐2,098) | 7 (1‐140) | <0.001 |
| Donor | |||
| Donor age (years) | 50 (2‐84) | 31 (18‐58) | <0.001 |
| Donor sex (n, % male) | 60 (54.5) | 46 (40) | 0.04 |
| Donor BMI (kg/m2) | 23.4 (14.8‐29.8) | 21.9 (15.4‐29.8) | <0.001 |
| Donor steatosis > 10% (n, %) | 18 (16.4) | 4 (3.5) | 0.001 |
Note: Data are presented as n (%) or median (range).
eGFR was based on the Modification of Diet in Renal Disease equation.
Organ support: either cardiovascular, ventilatory, or renal replacement therapy.
TABLE 4.
Perioperative Outcomes of LDLT and DDLT Groups
| DDLT Group (n = 110) | LDLT Group (n = 115) | P Value | |
|---|---|---|---|
| Recipient | |||
| Graft weight (g) | 1,232.5 (365‐2,180) | 600 (340‐970) | <0.001 |
| GRWR (%) | 1.77 (0.57‐3.95) | 0.9 (0.5‐1.7) | <0.001 |
| Right‐lobe graft (n, %) | — | 111 (96.5) | — |
| With MHV (n, %) | — | 106 (95.5) | |
| Cold ischemic time (minute) | 350.5 (101‐583) | 102 (39‐183) | <0.001 |
| Warm ischemic time (minute) | 49.5 (21‐107) | 51 (23‐86) | 0.96 |
| Operative time (minute) | 481.5 (270‐1,111) | 685 (435‐1,160) | <0.001 |
| Portal flow modulation (n, %) | 0 (0) | 4 (3.5) | — |
| Splenic artery ligation | — | 2 | |
| Splenic artery embolization | — | 1 | |
| Ligation of shunt | — | 1 | |
| ICU stay (days) | 4.5 (2‐20) | 4 (2‐142) | 0.475 |
| Hospital stay (days) | 21 (8‐325) | 22 (9‐354) | 0.301 |
| Hospital mortality (n, %) | 2 (1.8) | 4 (3.5) | 0.720 |
| Overall complication (n, %) | 86 (78.2) | 69 (60) | 0.004 |
| Severe postoperative complication* (n, %) | 0.025 | ||
| 3a | 25 (22.7) | 20 (17.4) | |
| 3b | 13 (11.8) | 13 (11.3) | |
| 4a | 8 (7.3) | 2 (1.7) | |
| 4b | 0 (0) | 1 (0.9) | |
| Specific complications (n, %) | |||
| Hepatic artery thrombosis | 1 (0.9) | 2 (1.7) | 1.000 |
| Portal vein thrombosis | 3 (2.7) | 0 (0) | 0.114 |
| Hepatic vein/IVC thrombosis | 2 (1.8) | 1 (0.9) | 0.485 |
| Biliary complications | 7 (6.4) | 9 (7.8) | 0.670 |
| Time to renal function recovery (days) | |||
| All patients | 5 (1‐322) | 4 (1‐151) | 0.089 |
| HRS patients only | 13 (1‐143) | 11 (1‐151) | 0.560 |
| Renal function recovery of all patients (n, %) | |||
| By 1 month | 65 (59.1) | 89 (77.4) | 0.003 |
| By 3 months | 82 (74.5) | 99 (86.1) | 0.029 |
| By 6 months | 86 (78.2) | 102 (88.7) | 0.033 |
| Renal function recovery of HRS patients (n, %) | |||
| By 1 month | 22 (40) | 31 (59.6) | 0.043 |
| By 3 months | 34 (61.8) | 40 (76.9) | 0.091 |
| By 6 months | 37 (67.3) | 41 (78.8) | 0.178 |
| Need for permanent RRT (n, %) | 3 (2.7) | 5 (4.3) | 0.512 |
| Listed or had renal transplant (n, %) | — | — | — |
| Donor | |||
| Donor ICU stay (days) | — | 1 (1‐6) | — |
| Donor hospital stay (days) | — | 8 (5‐28) | — |
| Donor overall complication (n, %) | — | 20 (17.4) | — |
| Donor postoperative complication* (n, %) | — | ||
| Grade 3 | — | 5 (4.3) | |
| Grade 4 | — | 0 (0) | |
Note: Data are presented as n (%) or median (range).
Clavien‐Dindo classification.
Abbreviation: IVC, inferior vena cava.
Donor Outcomes
The median time required for living donor workup from blood test to transplant was 3 (0‐59) days. Compared with deceased donors, living donors were younger, had lower BMI, and consisted of more female donors (Table 3). There was no donor death. The overall donor complication rate was 17.4% and 5 (4.3%) donors had grade 3 complication (Table 4). Two donors had endoscopic dilatation and stenting for bile duct stricture, 2 had image‐guided drainage of pleural effusion and intra‐abdominal collection, and 1 had bile leakage requiring endoscopic stenting and image‐guided drainage of subphrenic abscess.
Survival After Transplant
In ITT‐OS, the risk of mortality was the highest in the early period after listing. On the other hand, as illustrated in Fig. 3A,B, this was not observed in survival after transplant. Figure 3A,B showed the patient survival after LDLT versus DDLT for recipients with MELD > 25 and recipients with both MELD > 25 and HRS. The 1‐year, 3‐year, and 5‐year patient survival rates were 93.9% versus 90.9%, 92.2% versus 85.4%, and 88% versus 85.4% were comparable between LDLT and DDLT groups for recipients with MELD > 25 (P = 0.279) (Fig. 3A). There was no difference in patient survival between for recipients with MELD > 25 and HRS between the LDLT and DDLT group, the survival rates at 1, 3, and 5 years were 88.5% versus 85.5%, 84.6% versus 76.4%, and 77% versus 76.4%, respectively (P = 0.701). Early survival outcomes at 1, 4, 8, and 12 weeks after transplant in recipients with MELD > 25 and recipients with both MELD > 25 and HRS were similar between the 2 groups (Supporting Fig. S3A,B).
FIG. 3.
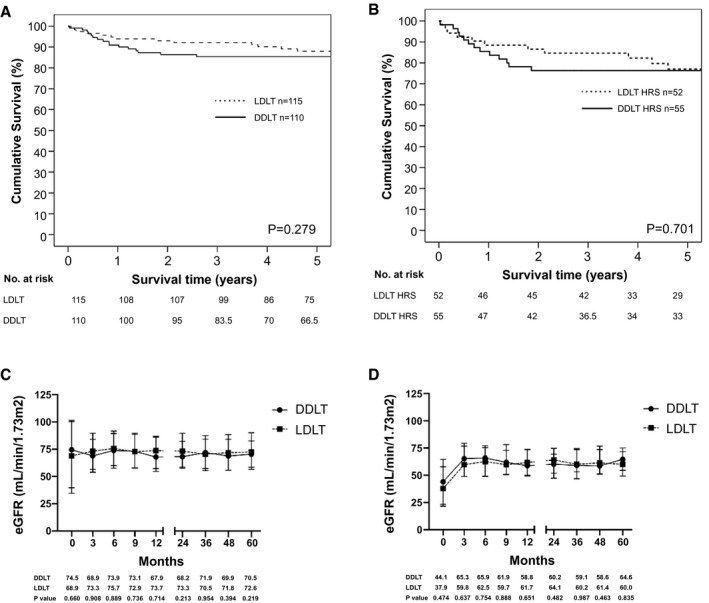
(A) Patient survival of recipients with MELD > 25 after LDLT versus DDLT. (B) Patient survival of recipients with MELD > 25 and HRS after LDLT versus DDLT. (C) eGFR of patients with MELD > 25 after LDLT versus DDLT. Data are shown as median and interquartile range. (D) eGFR of patients with MELD > 25 and HRS after LDLT versus DDLT. Data are shown as median and interquartile range.
Graft survival rates for MELD > 25 and both MELD > 25 and HRS were similar after LDLT and DDLT (Supporting Fig. S4A,B). The survival rates for recipients with MELD > 35 were also similar between the 2 groups (Supporting Fig. S5A,B).
Short‐Term and Long‐Term Renal Outcomes After Transplant
The median time to renal function recovery was less than 1 week in both groups. Patients who underwent LDLT had a higher chance for renal function recovery by 1 month (77.4% vs. 59.1%, P = 0.003), 3 months (86.1% vs. 74.5%, P = 0.029), and 6 months (88.7% vs. 78.2%, P = 0.033). If only patients with HRS were considered, the median time to renal function recovery was similar after DDLT versus LDLT (13 vs. 11 days, P = 0.56) but the chance of renal recovery was higher in patients who underwent LDLT by 1 month (59.6% vs. 40%, P = 0.043) (Table 4).
Concerning the long‐term renal outcomes, only 8 patients required permanent RRT, and none were listed or had renal transplant at the time of analysis (Table 4). There was no difference in median eGFR from 3 months to 5 years after LDLT and DDLT in recipients with MELD > 25 and recipients with both MELD > 25 and HRS (Fig. 3C,D). Similar findings were observed in recipients with MELD > 30, MELD > 30 and HRS, MELD > 35, and MELD > 35 and HRS (Supporting Fig. S6A‐D).
Predictors of Mortality and Graft Failure After Transplant
The proportionality of hazard assumption in Cox regression was confirmed using Schoenfeld’s global test (P = 0.783). In multivariable analyses, the following were associated with a higher risk of mortality and graft failure: an older recipient age (mortality HR, 1.055 [1.013‐1.098]; P = 0.008; graft failure HR, 1.054 [1.012‐1.108]; P = 0.011), higher MELD at transplant (mortality HR, 1.056 [1.007‐1.107]; P = 0.025; graft failure HR, 1.057 [1.008‐1.108]; P = 0.023), HCC (mortality HR, 3.83 [1.422‐10.315]; P = 0.008; graft failure HR, 3.718 [1.376‐10.046]; P = 0.01), a higher donor BMI (mortality HR, 1.145 [1.009‐1.299]; P = 0.036; graft failure HR, 1.146 [1.01‐1.3]; P = 0.035), and occurrence of postoperative complication (mortality HR, 1.286 [1.048‐1.578]; P = 0.016; graft failure HR, 1.285 [1.047‐1.577]; P = 0.016). Transplant type (LDLT vs. DDLT) was not associated with any difference in survival after transplant (Supporting Table S3). Further analyses performed by stratifying patients according to the MELD at listing showed that ITT‐LDLT had a consistent survival benefits (HR, 0.387‐0.552) across different MELD categories (MELD > 25‐30, >30‐35, and >35). If the survival was assessed from the time of transplant, the hazard of mortality was comparable between recipients of LDLT and DDLT (Table 5).
TABLE 5.
5‐Year Mortality and HR of Mortality According to MELD at Listing
| ITT‐LDLT 5‐Year Mortality | ITT‐DDLT 5‐Year Mortality | HR of Mortality ITT‐LDLT vs. ITT‐DDLT | 95% Confidence Interval | P Value | |
|---|---|---|---|---|---|
| At listing | |||||
| MELD > 25‐30 | 19.9% | 37.5% | 0.404 | 0.165‐0.980 | 0.047 |
| MELD > 30‐35 | 20.8% | 31.4% | 0.552 | 0.259‐0.990 | 0.049 |
| MELD > 35 | 36.9% | 69.2% | 0.387 | 0.243‐0.616 | <0.001 |
| LDLT 5‐Year Mortality | DDLT 5‐Year Mortality | HR of Mortality LDLT vs. DDLT | 95% Confidence Interval | P Value | |
|---|---|---|---|---|---|
| At transplant | |||||
| MELD > 25‐30 | 15.1% | 14.7% | 0.778 | 0.186‐3.257 | 0.732 |
| MELD > 30‐35 | 5.0% | 12.0% | 0.243 | 0.052‐1.148 | 0.074 |
| MELD > 35 | 15.3% | 18.5% | 0.789 | 0.273‐2.284 | 0.662 |
Discussion
In this study, we have shown consistent survival benefits of LDLT with ITT analysis and a reduction of waitlist mortality for patients with high MELD and HRS. The availability of living donors provided a significantly higher chance of transplant with significant reduction in the risk of mortality from the time of listing. Such benefits were consistently observed across different MELD categories. Early ITT‐OS analysis provided two important findings: (1) the steep decline observed in the curve indicated an extremely high risk of mortality without timely transplant in the early period after listing, especially for those with MELD > 35 and HRS; (2) the distinct alteration of slope of the survival curves showed that the true benefits of LDLT occurred within the first month after listing. The perioperative, survival, and renal outcomes for patients with high MELD and HRS were similar after LDLT and DDLT.
The results from the current study addressed two controversial issues regarding LDLT. Firstly, it challenged the notion that LDLT should not be considered in patients with MELD > 25 and HRS. The rationale was that LDLT in such settings carried an unacceptably high risk of morbidity and mortality because partial grafts were inadequate to reverse the pathophysiology of HRS and meet the metabolic demands of recipients with high MELD. Secondly, there have been concerns whether urgent donor workup protocol might pose additional psychological and physical risks to living donation.( 11 ) In the current study, hospital mortality was very low in patients with LDLT (4/115, 3.5%) and was comparable with patients with DDLT (2/110, 1.8%); 96.5% of patients with LDLT underwent transplantation using right‐lobe grafts with a median GRWR of 0.9%. There was also no difference in hospital/ICU stay and vascular or biliary complication after LDLT and DDLT. The 5‐year patient (88%) and graft survival (88%) of patients with MELD > 25 after LDLT was excellent and comparable with DDLT. These results reflected that a living donor graft indeed did not increase perioperative mortality and did not jeopardize the long‐term outcomes. The overall and severe perioperative complications were even lower in patients with LDLT despite having more patients with MELD > 35 and requiring ICU care before transplant. This is likely because LDLT allowed earlier access to transplant (median waiting time 7 days) in these patients who were critically ill.
In our cohort, 107/225 (47.6%) had HRS, 99/225 (44%) (47 DDLT, 52 LDLT) recipients of transplants had eGFR < 60 mL/min/1.73 m2 at time of transplant, and 21/107 (19.6%) required perioperative RRT. The HR of mortality showed substantial survival benefits (HR, 0.387‐0.552) in the ITT‐LDLT across different MELD strata. This is not surprising given that transplantation is perhaps the only way to reverse the pathophysiology of HRS. In addition, a living donor graft encompasses conditions for optimal graft quality, including young donor age, low BMI, minimal graft steatosis, and short cold ischemic time, even though the graft size is comparably “small.” With a 5‐year patient and graft survival rate of 77% in patients who had MELD > 25 and HRS, the current study demonstrated that LDLT was safe even in these patients. There was no difference in long‐term eGFR after LDLT and DDLT in patients with HRS. Overall, the time to renal function recovery was quicker after LDLT. In patients with HRS, the chance of renal recovery at 1 month after transplant was higher in LDLT. LDLT allows a timely access to liver transplantation (LT) and might hasten the renal function recovery in patients with HRS. Only 8 patients required permanent RRT, and none were listed or had renal transplant, indicating that renal recovery is possible irrespective of transplant type.
The availability of altruistic donors represents an important element to success. Among the 325 candidates listed for LT, 212/325 (65.2%) had a potential donor candidate. Because recipients were critically ill, all donor workup started simultaneously with recipient evaluation. Although the workup process for donors was expedited, it was not compromised. Donor safety is of paramount importance from a physical and psychological perspective. The donor workup process is protocol driven and the eligibility criteria remain unchanged. All doctors and nurses in the transplant team were trained to avoid donor coercion, and the option of DDLT is always offered and explained. As part of the standard protocol, a nontransplant surgeon and clinical psychologists will act as donor advocates and address the psychological concern of living donation. In the selected LDLT pair, a written approval from a statutory board is required. Nonetheless, the presence of living donors does not completely eliminate waitlist mortality. In our analysis, 28/91 (30.8%) patients’ conditions were irreversible, most commonly because of cerebral edema, MOF, and sepsis, and they would have been declined for transplant anyway.
Patients in the ITT‐LDLT group had a lower hazard of mortality across all MELD strata, and the higher the MELD, the higher the survival benefit. The survival curve began to plateau off after 1 year of listing and remained similar thereafter, indicating that waitlist and perioperative mortality was the most important cause of demise among these patients. In the current study, we have demonstrated that LDLT was feasible in patients with high MELD and HRS.
Not only did LDLT allow earlier access to transplant, but it also offered similar outcomes after transplant when compared with DDLT. Importantly, there was no live donor mortality in the current study, and the risk of grade 3 complications remained extremely low.
The strength of the current study includes a large cohort size with a long follow‐up time. There are several limitations. Firstly, this was a retrospective study of a single center. Secondly, there was likely selection bias because of the retrospective nature. A randomized trial is not feasible for obvious ethical reasons. This study was conducted in a region with a low deceased organ donation rate, such that the benefit of LDLT might be amplified. However, the shortage of deceased donor organs is universal, and the availability of LDLT will alleviate waitlist mortality. One should still be cautious, as patients with high MELD and HRS represent the highest‐risk group. Although the success of LDLT is multifactorial, the application of LDLT should not be limited by a certain MELD cutoff.( 20 ) With the advancement of surgical, anesthetic, and perioperative care, many centers that predominately perform DDLT also reported excellent outcomes after LDLT.( 5 , 10 , 27 ) Lastly, it is worth noting that right‐lobe grafts with inclusion of MHV were the predominant graft type in our study. Good venous outflow is as important as vascular inflow and graft size and contributed to the success of LDLT.( 20 )
In conclusion, for patients with MELD > 25 and HRS, the ITT‐LDLT approach reduced waitlist mortality by providing earlier access to transplant, particularly within the first month after listing when the mortality risk without transplant was the highest. A consistent survival benefit was observed across all MELD strata. LDLT was feasible and offered similar perioperative and long‐term outcomes to DDLT for high MELD and HRS patients. Donor workup under an expedited protocol was not associated with higher perioperative risk. LDLT should be considered for high MELD and HRS patients, and the application of LDLT should not be restricted by a MELD cutoff.
Author Contributions
T.C.‐L.W. was responsible for drafting of manuscript, conception, design, acquisition of data, and analysis and interpretation of data. J.Y.‐.Y.F., H.H.P., C.K.‐L.L., H.‐F.L., S.‐L.S., K.‐W.M., B.W.‐H.S., J.W.‐C.D., A.C.‐Y.C., T.‐T.C., and C.‐M.L. have all made significant contribution to conception, design, acquisition of data, and analysis and interpretation of data. They were also involved in patient recruitment and maintenance of the data set. All authors have participated in drafting and revising the manuscript. All authors have given final approval of the version to be published.
Supporting information
Fig S1‐S6
Supported by the General Research Fund (GRF 17115618) and Theme‐based Research Scheme (T12‐703‐19R) of the Research Grant Council, Hong Kong.
This paper was presented at the Liver Meeting 2019, American Association for the Study of Liver Diseases, Boston, MA, USA.
Potential conflict of interest: Nothing to report.
References
- 1. Feng S. Living donor liver transplantation in high Model for End‐Stage Liver Disease score patients. Liver Transpl 2017;23(Suppl. 1):S9‐S21. [DOI] [PubMed] [Google Scholar]
- 2. New York State Committee on Quality Improvement in Living Liver Donation . A report to: New York State Transplant Council and New York State Department of Health. New York: New York State Department of Health; 2002. [Google Scholar]
- 3. Ben‐Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim‐Schluger L, et al. Critical graft size in adult‐to‐adult living donor liver transplantation: impact of the recipient's disease. Liver Transpl 2001;7:948‐953. [DOI] [PubMed] [Google Scholar]
- 4. Testa G, Malago M, Nadalin S, Hertl M, Lang H, Frilling A, et al. Right‐liver living donor transplantation for decompensated end‐stage liver disease. Liver Transpl 2002;8:340‐346. [DOI] [PubMed] [Google Scholar]
- 5. Selzner M, Kashfi A, Cattral MS, Selzner N, McGilvray ID, Greig PD, et al. Live donor liver transplantation in high MELD score recipients. Ann Surg 2010;251:153‐157. [DOI] [PubMed] [Google Scholar]
- 6. Yi NJ, Suh KS, Lee HW, Shin WY, Kim J, Kim W, et al. Improved outcome of adult recipients with a high model for end‐stage liver disease score and a small‐for‐size graft. Liver Transpl 2009;15:496‐503. [DOI] [PubMed] [Google Scholar]
- 7. Chok K, Chan SC, Fung JY, Cheung TT, Chan AC, Fan ST, et al. Survival outcomes of right‐lobe living donor liver transplantation for patients with high Model for End‐stage Liver Disease scores. Hepatobiliary Pancreat Dis Int 2013;12:256‐262. [DOI] [PubMed] [Google Scholar]
- 8. Jiang L, Yan L, Tan Y, Li B, Wen T, Yang J, et al. Adult‐to‐adult right‐lobe living donor liver transplantation in recipients with hepatitis B virus‐related benign liver disease and high model end‐stage liver disease scores. Surg Today 2013;43:1039‐1048. [DOI] [PubMed] [Google Scholar]
- 9. Li H, Li B, Wei Y, Yan L, Wen T, Xu M, et al. Outcome of using small‐for‐size grafts in living donor liver transplantation recipients with high model for end‐stage liver disease scores: a single center experience. PLoS ONE 2013;8:e74081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Humar A, Ganesh S, Jorgensen D, Tevar A, Ganoza A, Molinari M, et al. Adult living donor versus deceased donor liver transplant (LDLT versus DDLT) at a single center: time to change our paradigm for liver transplant. Ann Surg 2019;270:444‐451. [DOI] [PubMed] [Google Scholar]
- 11. Levitsky J, Gordon EJ. Living donor liver transplantation when deceased donor is not possible or timely: case examples and ethical perspectives. Liver Transpl 2020;26:431‐436. [DOI] [PubMed] [Google Scholar]
- 12. Yoshizumi T, Taketomi A, Soejima Y, Uchiyama H, Ikegami T, Harada N, et al. Impact of donor age and recipient status on left‐lobe graft for living donor adult liver transplantation. Transpl Int 2008;21:81‐88. [DOI] [PubMed] [Google Scholar]
- 13. Chok KS, Fung JY, Chan AC, Dai WC, Sharr WW, Cheung TT, et al. Comparable short‐ and long‐term outcomes in living donor and deceased donor liver transplantations for patients with model for end‐stage liver disease scores ≥35 in a hepatitis‐B endemic area. Ann Surg 2017;265:173‐177. [DOI] [PubMed] [Google Scholar]
- 14. Chok KS, Fung JY, Chan SC, Cheung TT, Sharr WW, Chan AC, et al. Outcomes of living donor liver transplantation for patients with preoperative type 1 hepatorenal syndrome and acute hepatic decompensation. Liver Transpl 2012;18:779‐785. [DOI] [PubMed] [Google Scholar]
- 15. Lee JP, Kwon HY, Park JI, Yi NJ, Suh KS, Lee HW, et al. Clinical outcomes of patients with hepatorenal syndrome after living donor liver transplantation. Liver Transpl 2012;18:1237‐1244. [DOI] [PubMed] [Google Scholar]
- 16. Goldaracena N, Marquez M, Selzner N, Spetzler VN, Cattral MS, Greig PD, et al. Living vs. deceased donor liver transplantation provides comparable recovery of renal function in patients with hepatorenal syndrome: a matched case‐control study. Am J Transplant 2014;14:2788‐2795. [DOI] [PubMed] [Google Scholar]
- 17. Wong F. The evolving concept of acute kidney injury in patients with cirrhosis. Nat Rev Gastroenterol Hepatol 2015;12:711‐719. [DOI] [PubMed] [Google Scholar]
- 18. Sanyal AJ, Boyer T, Garcia‐Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized, prospective, double‐blind, placebo‐controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 2008;134:1360‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martín‐Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology 2008;134:1352‐1359. [DOI] [PubMed] [Google Scholar]
- 20. Wong TC, Fung JYY, Cui TYS, Sin SL, Ma KW, She BWH, et al. The risk of going small: lowering GRWR and overcoming small‐for‐size syndrome in adult living donor liver transplantation. Ann Surg 2020. Mar 20. 10.1097/SLA.0000000000003824. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21. Department of Health . The Human Organ Transplant Board. https://www.dh.gov.hk/english/links/links_hot.html. Published November 6, 2019. Accessed April 30, 2020. [Google Scholar]
- 22. Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg 2007;94:78‐86. [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247‐254. [DOI] [PubMed] [Google Scholar]
- 24. Gehan EA. A generalized Wilcoxon test for comparing arbitrarily singly‐censored samples. Biometrika 1965;52:203‐224. [PubMed] [Google Scholar]
- 25. Finkelstein DM, Goggins WB, Schoenfeld DA. Analysis of failure time data with dependent interval censoring. Biometrics 2002;58:298‐304. [DOI] [PubMed] [Google Scholar]
- 26. Sasaki K, Firl DJ, McVey JC, Schold JD, Iuppa G, Diago Uso T, et al. Elevated risk of split‐liver grafts in adult liver transplantation: statistical artifact or nature of the beast? Liver Transpl 2019;25:741‐751. [DOI] [PubMed] [Google Scholar]
- 27. Samstein B, Smith AR, Freise CE, Zimmerman MA, Baker T, Olthoff KM, et al. Complications and their resolution in recipients of deceased and living donor liver transplants: findings from the A2ALL cohort study. Am J Transplant 2016;16:594‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S6


