Abstract
Recent studies in alcohol use disorders (AUDs) have demonstrated some connections between carnitine metabolism and the pathophysiology of the disease. In this scoping review, we aimed to collate and examine existing research available on carnitine metabolism and AUDs and develop hypotheses surrounding the role carnitine may play in AUD. A scoping review method was used to search electronic databases in September 2019. The database search terms used included “alcohol, alcoholism, alcohol abuse, alcohol consumption, alcohol drinking patterns, alcohol‐induced disorders, alcoholic intoxication, alcohol‐related disorders, binge drinking, Wernicke encephalopathy, acylcarnitine, acetyl‐l‐carnitine, acetylcarnitine, carnitine and palmitoylcarnitine.” The inclusion criteria included English language, human‐based, AUD diagnosis and measured blood or tissue carnitine or used carnitine as a treatment. Of 586 studies that were identified and screened, 65 underwent abstract review, and 41 were fully reviewed. Eighteen studies were ultimately included for analysis. Data were summarized in an electronic data extraction form. We found that there is limited literature available. Alcohol use appears to impact carnitine metabolism, most clearly in the setting of alcoholic cirrhosis. Six studies found carnitine to be increased in AUD, of which 5 were conducted in patients with alcoholic cirrhosis. Only 3 placebo‐controlled trials were identified and provide some support for the use of carnitine in AUD to decrease cravings, anhedonia, and withdrawal and improve cognition. The increase in plasma carnitine in alcoholic cirrhosis may be related to disordered fatty acid metabolism and oxidative stress that occurs in AUD. The multiple possible therapeutic effects carnitine could have on ethanol metabolism and the early evidence available for carnitine supplementation as a treatment for AUD provide a foundation for future randomized control trials of carnitine for treating AUD.
Keywords: Alcohol Use Disorder, Carnitine, Scoping Review
Alcohol remains a high cause of morbidity and mortality with 132.6 million disability‐adjusted life years lost (DALYs) and 3 million deaths estimated worldwide in 2016 (Global Status Report on Alcohol and Health, 2018, 2019). A significant proportion of these alcohol‐related harms are sustained by persons living with an alcohol use disorder (AUD). As defined by the DSM‐5, an AUD is a problematic pattern of alcohol use leading to clinically significant impairment or distress (American Psychiatric Association, 2013).The estimated point prevalence of AUDs worldwide in 2016 in people aged 15 and over was 5.1% (over 283 million people; Global Status Report on Alcohol and Health, 2018, 2019).
Prior to the mid‐20th century, there were limited targeted options for the treatment of alcohol use disorders. Since the discovery of disulfiram in 1948, there have been numerous developments in the conceptual frameworks underlying addiction to alcohol and pharmacologic strategies for managing alcohol use disorders (Nutt et al., 2015; Zindel and Kranzler, 2014). After the establishment of the dopamine positive reinforcement theory of addiction in the 1970s, multiple nondopaminergic systems have been proposed to play a role in alcohol addiction such as the endogenous opioid positive reinforcement and corticotropin‐releasing hormone negative reinforcement theories (Herz, 1997; Koob, 2011; Nutt et al., 2015). There are currently 3 approved pharmocotherapy treatments available for AUD: disulfiram, acamprosate, and naltrexone (Reus et al., 2018). Disulfiram inhibits aldehyde dehydrogenase, preventing the breakdown of acetaldehyde and resulting in an adverse reaction if alcohol is consumed. (Skinner et al., 2014) Acamprosate modulates neural transmission from GABA and NMDA receptors to reduce withdrawal symptoms, (Kalk and Lingford‐Hughes, 2014) whereas naltrexone, an opioid receptor antagonist, interferes with the reward pathway activated in AUD. Acamprosate and naltrexone are the preferred pharmacotherapies for AUD (Jonas et al., 2014; Reus et al., 2018) although are contraindicated in the presence of severe renal impairment and severe liver impairment, respectively, both of which can be complications of AUD (Reus et al., 2018). Other pharmacologic targets under research cover a wide range of mechanisms of action including dopamine, GABA, glutamate, opioid, and serotonin pathways (Shen, 2018; Zindel and Kranzler, 2014). Ultimately, current guidelines recommend the use of both pharmacologic and nonpharmacological options (including psychotherapy and/or community‐based peer support) in the management of AUDs (Reus et al., 2018).
Despite the above‐mentioned progress, there remain calls to continue developing new medications to treat AUDs due to the modest efficacy of the options available (Allenby and Falcone, 2017; Jones et al., 2015; Nutt et al., 2015; Shen, 2018; Zindel and Kranzler, 2014). Recent developments in the study of carnitine as a therapeutic agent raises possible new connections with the pathophysiology of AUD. Since the year 2000, multiple studies and subsequent systematic reviews have pointed to the potential therapeutic applications of carnitine for diseases impacting cognition including dementia and hepatic encephalopathy (Hudson and Tabet, 2003; Martí‐Carvajal et al., 2019). Additionally, investigations into the pathophysiology of alcohol‐mediated fatty liver disease have found an association between plasma deficiencies in carnitine and chronic alcohol consumption (Kępka et al., 2013, 2019).
Carnitine
A role for carnitine in energy metabolism has long been established. Carnitine is responsible for the movement of long‐chain fatty acids (FAs) into the mitochondria for β‐oxidation through the action of carnitine palmitoyltransferase‐1 (CPT1) (Longo et al., 2016). It also plays an essential role in trafficking and scavenging of acyl groups within the cell. For example, in the peroxisome where very‐long‐chain FAs are preferentially metabolized, carnitine shuttles the fatty acyl end products of beta oxidation between the peroxisome and the mitochondria (Steiber et al., 2004). As a scavenger, carnitine binds acyl‐CoA residues and intermediaries of protein and FA metabolism, which can then be excreted or circulated to another part of the body. This decreases the availability of free fatty acyls, reducing the incidence of lipid peroxidation. The carnitine bound acyl‐CoA residues within the mitochondria modulate intracellular concentrations of free CoA and are thought to play a key role in maintaining the acyl‐CoA/CoA ratio, an important regulator of energy metabolism (Brass, 2003; Longo et al., 2016; Steiber et al., 2004).
Carnitine has also been found to decrease markers of oxidative stress (Calabrese et al., 2001; Lee et al., 2014; Sener et al., 2004), scavenge free radicals (Ribas et al., 2014), bind organic acids for removal, chelate cytosolic iron (Gülçin, 2006), and increase antioxidant enzyme activity (Calabrese et al., 2001; Gülçin, 2006; Lee et al., 2014). Finally, carnitine has been shown to support the role of glutathione in oxidative stress by increasing GSH reductase and arginase activities (Scapagnini et al., 2002). All of these functions of carnitine are critical in AUDs, where inflammation and oxidative stress are high and excess acetyl‐CoA is produced from ethanol (EtOH) metabolism.
EtOH Metabolism
The metabolism of EtOH has a profound impact on normal oxidative metabolism and can lead to hepatic lipid accumulation. The oxidative metabolism of EtOH by alcohol dehydrogenase (ADH) and cytochrome P450 2E1 (CYP2E1) leads to the rapid accumulation of NADH, changing the NAD+/NADH ratio, effectively downregulating FA oxidation and leading to a metabolic shift favoring FA synthesis (Donohue Jr., 2007). Acetaldehyde produced by ADH is further metabolized to acetate by acetaldehyde dehydrogenase. Acetyl‐CoA is formed from acetate; however, since oxidative metabolism is inhibited, the excess acetyl‐CoA is converted to FA‐CoA, adding to lipid accumulation. EtOH also activates sterol regulatory binding protein‐1C (SREBP‐1C), a transcription factor which regulates lipogenic gene expression in the liver, further contributing to lipid accumulation (Osna et al., 2017).
In addition to the profound shift in lipid metabolism, EtOH metabolism by CYP2E1 produces high amounts of reactive oxygen species (ROS) which is an important mechanism in oxidative stress‐induced liver injury in AUD (Cederbaum, 2012; Osna et al., 2017).
Carnitine in AUD
Evidence in rodent models suggests that carnitine supplementation may mitigate the deleterious effects of EtOH metabolism. Carnitine supplementation has been shown to decrease triacylglycerol (TAG) synthesis and hepatic lipid accumulation that normally occur in EtOH treated rodents (Bykov and Belkovets, 2004; Bykov et al., 2003; Murad et al., 1977; Sachan and Rhew, 1983; Sachan et al., 1984). It has also been shown to decrease EtOH‐induced inflammation (Bykov et al., 2003) and protect against oxidative damage in the liver, serum and brain (Arafa and Sayed‐Ahmed, 2003; Augustyniak and Skrzydlewska, 2009, 2010; Best and Laposata, 2003; Bykov et al., 2003; Dobrzyńska et al., 2010; Haorah et al., 2013). Co‐administration of either carnitine or acetyl‐carnitine with EtOH decreases fatty acid ethyl ester synthesis (Calabrese and Rizza, 1999; Calabrese et al., 2001), an esterification product of EtOH and FAs that has been implicated in the organ damage induced by chronic alcohol use (Doyle et al., 1994; Laposata and Lange, 1986).
The cognitive deficits and neuropathy that can occur with AUD are thought to be a result of mitochondrial dysfunction in the brain. The accumulation of acetaldehyde in the brain has been proposed to contribute to the development of alcohol dependence (Deng and Deitrich, 2008). Early studies by Murad and colleagues (1977) demonstrated that carnitine supplementation in EtOH treated rats decreased EtOH intake and withdrawal symptoms and proposed that carnitine deficiency may play a role in developing alcohol physical dependence (Murad et al., 1977). Carnitine has been shown to support normal mitochondrial function, and decreases oxidative stress in the brain of EtOH‐treated rodents (Haorah et al., 2009, 2013). Carnitine also supports acetylcholine formation, by donating an acetyl group and has been found to be neuroprotective in Alzheimer’s disease and aging (Pettegrew et al., 1995).
Considering the evidence available in rodent models of addiction and the continuing need for effective treatments for AUD, this research topic lends particularly well to a scoping review. A scoping review is meant to broadly map the existing research on a particular topic. One limitation of a scoping review is there is no critique of the quality of studies included (Peterson et al., 2017). Therefore, we conducted a scoping review to consolidate the existing evidence surrounding AUDs and carnitine metabolism in humans. The objectives of this review are to (i) propose biologically plausible explanation(s) of how carnitine metabolism could contribute to the development and/or maintenance of an AUD and (ii) identify limitations and gaps in the literature available surrounding carnitine metabolism defects and AUDs as a basis for future study.
Materials and Methods
This scoping review was conducted according to the 5 stage methodology described by Khalil and colleagues (2016). In October 2019, we searched the following databases: Ovid MEDLINE, Embase + Embase Classic, PsycINFO, and EBM Reviews—Cochrane Central Register of Controlled Trials and Web of Science. The protocol and search strategy are available in supplementary materials. The database search terms used included “alcohol, alcoholism, alcohol abuse, alcohol consumption, alcohol drinking patterns, alcohol‐induced disorders, alcoholic intoxication, alcohol‐related disorders, binge drinking, wernicke encephalopathy, acylcarnitine, acetyl‐l‐carnitine, acetylcarnitine, carnitine and palmitoylcarnitine.” Inclusion criteria included English language, primary medical and biochemical research literature, and studies conducted in humans which measured blood and/or tissue carnitine levels and/or provided carnitine treatment. Exclusion criteria included studies evaluating other substance use disorders, carnitine and mood disorders, non‐English studies, studies conducted in cell lines or rodent studies and conference abstracts.
Phase 1 and 2 review of titles and abstracts were conducted by JGS and ABB. Paper review and data extraction were conducted by ABB, JGS, and AB. Data extraction was conducted using an electronic data extraction form. Stage 3 (Back Searching): Analysis of the reference lists of all articles for consideration for inclusion (JGS, ABB).
Our database search identified 586 studies. Of these, 65 underwent abstract screening. Twenty‐five abstracts were removed for the following reasons: nonhuman (16), non‐English (6), and not primary research (2) and not alcohol‐related (1). 40 articles underwent full review.
Results
Search Strategy
From the original search, 40 articles underwent full review. We excluded 23 papers for the following reasons: no alcohol‐specific data (6), duplicate (3), not primary research (1), no paper available (4), and non‐English (1). We also identified 2 clinical trials; however, no data were available at the time of review. Six (6) studies used primary human cell culture to evaluate the effects of EtOH and carnitine on metabolism and cellular function in primary human brain (3), lung (1), erythrocytes (1) cell culture and ex vivo liver samples (1) and were excluded from the final analysis. Back searching identified 1 additional study (Fig. 1).
Fig. 1.
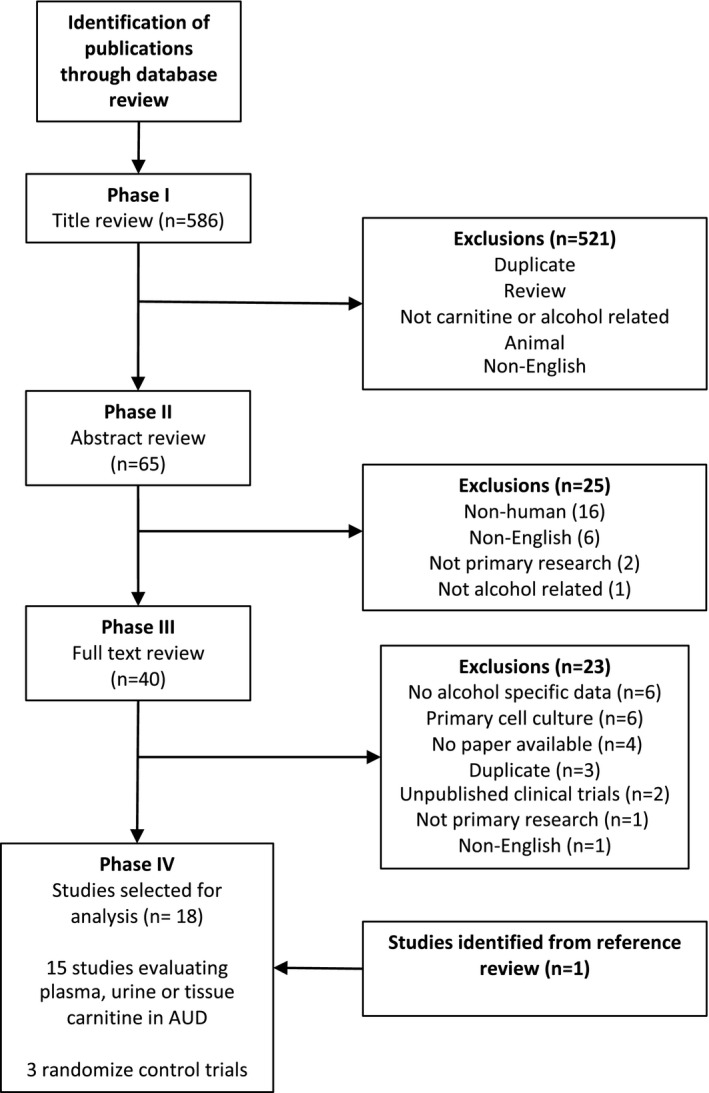
Flowchart of literature review.
Study Characteristics
Of the 18 relevant studies identified, 9 were conducted among patients in an AUD program either in hospital (Fuller and Hoppel, 1988; Glass et al., 1989; Harper et al., 1993; Kępka et al., ,2013, 2016, 2019; Martinotti et al., ,2010, 2011; Tempesta et al., 1990) or as outpatients (Martinotti et al., ,2010, 2011; Tempesta et al., 1990). In 3 studies, patients were in hospital due to liver disease (Ascha et al., 2016; Rudman et al., 1977; Suzuki, 2016) and 1 study recruited patients from a hepatological outpatient clinic (Krähenbühl and Reichen, 1997). One study was conducted in patients in hospital; however, it was not clearly stated whether they were admitted for AUD or alcoholic liver disease (Fuller and Hoppel, 1983). Four studies did not provide details in this regard (Alonso De La Pena et al., 1990; Amodio et al., 1990; Cooper et al., 1987; de Sousa et al., 1988). The majority of studies were conducted in Europe (n = 9) (Alonso De La Pena et al., 1990; Amodio et al., 1990; Harper et al., 1993; Kępka et al., ,2013, 2016, 2019; Krähenbühl and Reichen, 1997; Martinotti et al., ,2010, 2011; Tempesta et al., 1990), with 4 in the United States (Ascha et al., 2016; Fuller and Hoppel, 1983, 1988; Rudman et al., 1977), 3 from the United Kingdom (Cooper et al., 1987; Glass et al., 1989; de Sousa et al., 1988), and 1 from Asia (Suzuki, 2016).
Of the 18 relevant papers, we identified 15 studies which measured carnitine levels (Table S1). Of these 15 studies, 14 measured plasma or serum carnitine, and a subset measured tissue carnitine including liver carnitine (n = 3), urinary carnitine (n = 5), and muscle carnitine (n = 2). Three (3) studies were placebo‐controlled supplement trials; however, none of these studies measured plasma or tissue carnitine levels (Table S3).
Of the studies 14 studies which evaluated plasma, tissue or urinary carnitine levels, 9 of these studies were in patients with evidence of liver disease (Alonso De La Pena et al., 1990; Amodio et al., 1990; Ascha et al., 2016; Cooper et al., 1987; Fuller and Hoppel, 1983, 1988; Krähenbühl and Reichen, 1997; Rudman et al., 1977; Suzuki, 2016) of which all but one (Cooper et al., 1987) was conducted in patients with alcoholic cirrhosis.
Plasma Carnitine in AUD
Table S1 presents the studies which evaluated tissue and plasma carnitine in AUDs. Six (Alonso De La Pena et al., 1990; Cooper et al., 1987; Fuller and Hoppel, 1983, 1988; Glass et al., 1989; Krähenbühl and Reichen, 1997) studies demonstrated higher plasma carnitine levels compared to control values, although one of these was not statistically significant (Cooper et al., 1987); 3 were not significantly different (Alonso De La Pena et al., 1990; Amodio et al., 1990; Harper et al., 1993; de Sousa et al., 1988); and 3 found levels to be reduced (Ascha et al., 2016; Kępka et al., 2013; Rudman et al., 1977). One study did not have a control group (Suzuki, 2016). Details on the analytics of carnitine analysis and additional study data can be found in Table S2.
De la Pena et al. assessed plasma carnitine levels in a large cohort of patients with AUDs with varying degrees of liver disease. They found the highest levels of carnitine in cirrhotic patients, which is consistent with others (Fuller and Hoppel, 1983, 1988). However, they also found evidence of hypocarnitinemia which occurred predominantly in noncirrhotic patients with hepatitis (Alonso De La Pena et al., 1990). Cooper et al. reported plasma carnitine in patients with AUD and varying degrees of hepatic injury to be higher than controls. However, this was not significant due to large variability in the alcoholic cirrhotic population (Cooper et al., 1987). We did not categorize the study by Amodio et al. as evidence that patients with alcohol use had elevated plasma carnitine levels despite its report that total, esterified, short chain and acetylcarnitine were physiologically higher in cirrhotic patients. Notably, the statistical comparisons in this paper were only done with cirrhotic patients with AUDs and not with the control population (Amodio et al., 1990).
Several factors may contribute to higher levels of carnitine in patients with AUDs. Of the 6 studies which found plasma carnitine to be increased (Alonso De La Pena et al., 1990; Cooper et al., 1987; Fuller and Hoppel, 1983, 1988; Glass et al., 1989; Krähenbühl and Reichen, 1997), 5 were conducted in patients with alcoholic cirrhosis (Alonso De La Pena et al., 1990; Cooper et al., 1987; Fuller and Hoppel, 1983, 1988; Krähenbühl and Reichen, 1997), 1 did not report on the presence of liver disease (Glass et al., 1989), and 1 study reported evidence of hepatic disease which ranged from mild fatty liver to decompensated cirrhosis with encephalopathy and ascites (Cooper et al., 1987).
Of the 3 studies which saw no difference in plasma carnitine in patients with AUDs compared to the control population, patients had no evidence of cirrhosis (Alonso De La Pena et al., 1990; Harper et al., 1993; de Sousa et al., 1988) with varying degrees of alcoholic steatosis and hepatitis (Alonso De La Pena et al., 1990; de Sousa et al., 1988). De la Pena et al. only saw carnitine values similar to the control in populations in groups with less severe hepatic disease (slight hepatomegaly with intermittent hepatic synthetic dysfunction and/or liver enzyme elevations). However, as noted above, plasma carnitine was increased in their study’s patients with alcohol‐related steatosis, hepatitis, and cirrhosis (Alonso De La Pena et al., 1990). Suzuki et al. did not have a control population, and although they concluded the observed values in the alcoholic cirrhosis population were within the normal range based on literature, the mean carnitine values were similar to the hypercarnitinemia observed in alcoholic cirrhosis in other studies (Suzuki, 2016).
Three studies found plasma carnitine levels to be decreased compared to their control population. However due to varying study designs, this made them challenging to compare. In 1 of the studies which reported lower plasma carnitine levels in AUD, the level of liver disease was not specified (Kępka et al., 2013). Ascha et al. reported lower carnitine levels in alcoholic cirrhosis patients; however, this was only evident in the octanoylcarnitine species and the control group was a patient population with acute decompensated cirrhosis (56). Rudman et al. reported reduced serum carnitine in alcoholic cirrhotic patients compared to controls. This patient population was hospitalized with advanced alcoholic cirrhosis and had evidence of anorexia and malnutrition (Rudman et al., 1977).
The majority of studies measured total, free and acylcarnitine levels, except for Ascha et al. which used mass spectrometry to quantify specific acylcarnitine species (Ascha et al., 2016). Most studies found that all carnitine pools followed the same trend. In the studies that found carnitine to be increased, total carnitine was not changed in 2 studies (Amodio et al., 1990; Krähenbühl and Reichen, 1997) and 1 study reported no change in acylcarnitines but an increase in total and free carnitines (Glass et al., 1989). Acetylcarnitine pools were evaluated in 2 studies of alcoholic cirrhotic patients and were not found to be different to the control group (Amodio et al., 1990; Ascha et al., 2016).
The duration of alcohol dependence and dose of alcohol may also play a role in carnitine levels; however, this was only reviewed in 1 study. Kępka and colleagues (2019) found that free carnitine was higher in patients who consumed larger doses of alcohol (275 to 700 g) compared to lower doses (75 to 125 g); however, total carnitine was lower with a longer duration of alcohol use (20 to 30 years vs. 2 to 7 years) (Kępka et al., 2019).
Urinary Carnitine in AUD
Urinary carnitine was measured in patients with AUDs in 5 studies (Fuller and Hoppel, 1983; Kępka et al., 2016; Krähenbühl and Reichen, 1997; Rudman et al., 1977; de Sousa et al., 1988). Two studies found urinary carnitine excretion to be higher in patients with AUDs (Fuller and Hoppel, 1983; Kępka et al., 2016), 2 found no difference compared to control values (Krähenbühl and Reichen, 1997; de Sousa et al., 1988), and 1 study in patients with advanced alcoholic cirrhosis in the presence of anorexia found urinary carnitine to be decreased compared to controls (Rudman et al., 1977). Notably, in 1 of the 2 studies that found urinary excretion of carnitine to be increased, this difference was not significant due to considerable variability (Fuller and Hoppel, 1983).
Tissue Carnitine
Liver carnitine was evaluated in 3 studies, with varying results (Harper et al., 1993; Rudman et al., 1977; de Sousa et al., 1988). Two of these studies showed no difference in plasma carnitine levels, with some evidence of liver disease. De Sousa et al. found no difference in plasma total, free or acylcarnitine levels between control and patients with AUD (with no evidence of cirrhosis); however, total and free carnitines were decreased in the liver (de Sousa et al., 1988). Harper et al. found no difference in liver carnitine compared to control; however, liver carnitine significantly increased after 9 to 11 days of abstinence (Harper et al., 1993). In contrast, Rudman et al. evaluated postmortem liver carnitine levels in 6 patients with alcoholic cirrhosis who had been enrolled in the earlier phase of the study measuring serum carnitine but died due to a complication of their liver disease compared to 8 noncirrhotic controls who had died due to an acute illness of 1 to 3 days. Postmortem hepatic carnitine was reduced compared to controls (Rudman et al., 1977).
Muscle carnitine was evaluated in 2 studies (Rudman et al., 1977; de Sousa et al., 1988). De Sousa et al. found skeletal muscle total and free carnitine to be increased in patients with AUD, with normal plasma and urinary carnitine and decreased liver carnitine. The authors suggested the high muscle carnitine was due to type 1 fiber enrichment since type 2 fiber atrophy was evident (de Sousa et al., 1988). Rudman et al. also evaluated postmortem muscle, brain, heart, and kidney carnitine in their study’s patients who died of alcoholic cirrhosis complications, all of which were decreased compared to the control population (Rudman et al., 1977).
Temporal Changes in Carnitine
Four studies evaluated changes in plasma and/or urinary carnitine before (or in the early stages of) and after a period of abstinence (Fuller and Hoppel, 1988; Harper et al., 1993; Kępka et al., 2016). Two studies observed no change (Fuller and Hoppel, 1988; Harper et al., 1993) and 1 study found carnitine decreased over time (Kępka et al., 2016). Fuller and Hoppel found no significant change in plasma carnitine after 3 weeks of sobriety in a subset of AUD patients without cirrhosis (Fuller and Hoppel, 1988). Harper and colleagues (1993) evaluated carnitine after 9 to 11 days of sobriety and also found no change in plasma levels. However, hepatic carnitine increased along with liver lipid density and patients had improved markers of synthetic liver function. In a study conducted by Kępka et al, total and free urinary carnitine decreased after 30 and 49 days of sobriety to levels similar to the control population (Kępka et al., 2016).
Carnitine Supplementation Studies
Three placebo‐controlled trials evaluated the effects of acetyl‐L‐carnitine (ALC) supplementation on anhedonia (Martinotti et al., 2011), alcohol cravings (Martinotti et al., 2010), memory and cognitive deficits (Tempesta et al., 1990) in AUD and are summarized in Table S3. ALC was effective in improving cognition (Tempesta et al., 1990), anhedonia (Martinotti et al., 2011), and reduced incidence of relapse and cravings (Martinotti et al., 2010) in AUD. Interestingly, the studies by Martinotti et al. found the greatest effect of ALC in the first 10 days of treatment, which was given by IV. The lack of effect for the remaining oral treatment period may have been due to lack of adherence to the study protocol as a total of 17 participants were considered noncompliant or related to small sample size. These trials had considerable loss to follow‐up and drop‐out rates which the authors suggested may have been related to the anhedonic patient population (Martinotti et al., 2010, 2011). In all 3 studies, ALC was also found to be safe and well tolerated which has been shown in other clinical trials for dementia, depression, and pain disorders (Evans et al., 2008; Sima et al., 2005; Veronese et al., 2018).
Discussion
The purpose of this review was to provide an overview of the existing evidence surrounding the association between AUD and carnitine metabolism. We identified 15 studies which measured blood and/or urine or tissue carnitine levels in alcoholics as well as 3 placebo‐controlled trials. Although there was variability across studies, it appears that AUD has an effect on body carnitine, and carnitine supplementation may support treatment.
Plasma and Tissue Carnitine are Altered in AUD
The main finding of this review is that AUDs alter plasma and tissue carnitine levels, particularly in the presence of alcoholic cirrhosis. Currently, the changes in plasma carnitine that occur in AUD are not well understood and warrant further research. There are several factors which could impact carnitine levels in AUD, and we have discussed them below.
Plasma carnitine was found to be increased compared to the control group in 5 (Alonso De La Pena et al., 1990; Cooper et al., 1987; Fuller and Hoppel, 1983, 1988; Krähenbühl and Reichen, 1997) of 8 (Alonso De La Pena et al., 1990; Amodio et al., 1990; Ascha et al., 2016; Cooper et al., 1987; Fuller and Hoppel, 1983, 1988; Krähenbühl and Reichen, 1997; Rudman et al., 1977) studies of patients with an AUD and cirrhosis. Of the remaining 3 studies, 2 (Amodio et al. and Suzuki et al.) had methodological aspects that confound the interpretation of plasma carnitine in patients with an AUD and cirrhosis. Finally, the eighth study notably found a decrease in plasma carnitine but was conducted in a population with advanced liver disease with evidence of malnutrition and anorexia (Rudman et al., 1977).
Following our review, we propose that increased plasma carnitine may be the result of disordered FA metabolism in alcohol use. As discussed previously, carnitine plays an essential role in intracellular and interorgan trafficking of fatty acids and buffering the acyl‐CoA/CoA ratio. In alcoholic liver disease, fatty acid synthesis and lipid deposition increases dramatically due to the inhibition of FA oxidation by excess NADH synthesis, the accumulation of acetyl‐CoA (precursor for FA synthesis) as a result of acetate metabolism and the upregulation of fatty acid synthesis machinery through the transcriptional activation of SREBP‐1c (Osna et al., 2017). It is possible that as alcoholic liver disease progresses and fatty acid metabolism becomes impaired, plasma and tissue carnitine increases. This increase in carnitine biosynthesis may be an adaptive mechanism to deal with the increase in fatty acyls and high levels of oxidative stress and inflammation in AUDs.
An increase in carnitine biosynthesis in AUD was previously proposed by Krähenbühl and Reichen (Krähenbühl and Reichen, 1997). The first step of carnitine biosynthesis is the formation of trimethyllysine in skeletal muscle. Protein turnover has been found to be increased in AUD patients (Marchesini et al., 1981), suggesting there may be increased trimethyllysine available for carnitine biosynthesis. Another yet to be explored possibility is that plasma carnitine is reflective of carnitine released from skeletal muscle as 95% of body carnitine resides in this tissue, and muscle wasting can occur in AUD (Steiner and Lang, 2015). However to the best of our knowledge, no studies have evaluated muscle and plasma carnitine in sarcopenia. De Sousa et al. found skeletal muscle carnitine to be increased in AUD; however, this may have been reflective of the type II fiber atrophy that was evident (de Sousa et al., 1988).
We also found evidence of hypocarnitinemia in advanced alcoholic liver disease (Alonso De La Pena et al., 1990; Fuller and Hoppel, 1983; Rudman et al., 1977). It is possible that the oxidative stress, malnutrition, liver damage, and muscle wasting that occur in more advanced liver disease impair the body’s ability to upregulate carnitine synthesis, while urinary reabsorption is reduced due to renal insufficiency. Together, these contribute to hypocarnitinemia in advanced disease. Rudman et al. demonstrated that carnitine supplementation in individuals with advanced alcoholic cirrhosis restored carnitine levels suggesting that carnitine is still a treatment option in later stages of disease (Rudman et al., 1977). More research needs to be done to better understand the underlying pathophysiology of changes in body carnitine stores in AUD.
Changes in urinary excretion of carnitine and carnitine reabsorption would also contribute to differences in plasma carnitine values. However, in the 5 studies that reported urinary carnitine, there was no observable trend (Fuller and Hoppel, 1983; Kępka et al., 2019; Krähenbühl and Reichen, 1997; Rudman et al., 1977; de Sousa et al., 1988). In patients that had elevated urinary carnitine, this returned to control levels after a period of abstinence (Kępka et al., 2019). It is possible that changes in urinary excretion could be an adaptive response to either conserve carnitine (increase reabsorption) or excrete excess acylcarnitines formed from an increase availability of acetyl‐CoA or ethyl esters. It has been proposed that increased urinary carnitine may be a result of impaired renal tubule function contributing to decrease carnitine reabsorption (Kępka et al., 2016) as alcohol abuse can impair renal reabsorption (Labib et al., 1989). In the current studies, carnitine renal excretion was decreased in cirrhotic patients with renal insufficiency (Fuller and Hoppel, 1983; Rudman et al., 1977). More research needs to be done to understand the role of the kidney in carnitine function in AUD.
A Role for Carnitine in Treating AUD
Based on the evidence summarized in this review, we propose that carnitine supplementation could play a supportive treatment role in the altered metabolic state induced by AUD by supporting oxidative metabolism, reducing oxidative stress as well as decreasing cravings and withdrawal.
Carnitine may Support Oxidative Metabolism and Decreasing Oxidative Stress in AUD
Excess acetyl‐CoA produced during alcohol metabolism contributes to hepatitis that can develop with an AUD. Carnitine, through the action of carnitine acetyltransferase, aids in the clearance of acetyl‐CoA by forming acetylcarnitine, which can leave the mitochondria and cell and be excreted in urine. Indeed, Adamo et al. found that carnitine administration during EtOH consumption in humans increased the clearance of acetylcarnitine in the urine and decreased acetate appearance in the blood (Adamo et al., 1988). It is possible that endogenous carnitine stores are inadequate in dealing with the influx of acetate and acetyl‐CoA produced following EtOH consumption and carnitine supplementation could prevent or reduce alcoholic liver disease, even in incidence where plasma carnitine is already increased. This is supported by rodent studies that found that carnitine supplementation decreases the incidence of fatty liver (Bykov and Belkovets, 2004; Bykov et al., 2003; Murad et al., 1977; Sachan and Rhew, 1983; Sachan et al., 1984), promotes the clearance of fatty acid ethyl esters (Calabrese and Rizza, 1999; Calabrese et al., 2001), decreases inflammation (Bykov et al., 2003), and protects against oxidative damage in liver, serum, and brain (Arafa and Sayed‐Ahmed, 2003; Augustyniak and Skrzydlewska, 2009, 2010; Bykov et al., 2003; Dobrzyńska et al., 2010; Haorah et al., 2013). A recent systematic review on carnitine supplementation in nonalcoholic fatty liver disease found carnitine to be a safe and effective treatment for fatty liver disease (De Guzman Boado et al., 2019).
Carnitine in the Management of Cravings, Anhedonia, and Withdrawal in AUD
In addition to supporting oxidative metabolism, there is evidence to suggest that carnitine may play a role in reducing cravings and withdrawal in AUD. This review identified 3 clinical trials evaluating the efficacy of acetyl‐L‐carnitine in AUD which demonstrated that carnitine improved anhedonia, reduced alcohol cravings and withdrawal symptoms and increased time to relapse. Early studies by Murad and colleagues (1977) demonstrated that carnitine supplementation in EtOH‐treated rats decreased EtOH intake and withdrawal symptom and proposed that carnitine deficiency may play a role in developing alcohol physical dependence (Murad et al., 1977), which was supported by others (Corbett and Leonard, 1984; Sachan et al., 1984). It should be noted however that this study used a very high dose of carnitine and it is unknown if this effect would be observed in humans using a standard carnitine dose (Murad et al., 1977). Mangano et al. also demonstrated that acetyl‐L carnitine supplementation reduced cravings and withdrawal symptoms in EtOH addicted rodents and decreased EtOH consumption (Mangano et al., 2000; Murad et al., 1977).
The mechanism by which carnitine may reduce withdrawal and cravings is not yet known. Carnitine crosses the blood–brain barrier through the transporter OCTN2 and accumulates in cells in the brain where it has been shown to play a role in providing FA for cellular membrane structure and supports acetylcholine synthesis (Nałȩcz et al., 2004; Wawrzeńczyk et al., 1995). Carnitine has also been shown to be effective in treatment for depression and Alzheimer’s among other neurological conditions (Evans et al., 2008; Pettegrew et al., 1995; Sima et al., 2005; Veronese et al., 2018).
With only 2 randomized control trials in AUD, it is difficult to draw conclusions on the effectiveness of carnitine supplementation in this population. Furthermore, the studies published by Martinotti et al. had a small sample size and only saw significant improvements following the initial 10 day intravenous treatment. (Martinotti et al., ,2010, 2011) Despite this, it is worth further evaluation as even a short duration of improvement may allow patients to remain alcohol free for longer.
Overall, the research in rodents and humans suggests that carnitine may be an effective, safe, and tolerable supportive treatment for individuals living with an AUD and undergoing treatment for the disorder. Future high‐quality placebo‐controlled trials are needed to evaluate the effectiveness of carnitine in reducing cravings, withdrawal, and dependence in AUD, and to determine if carnitine supplementation could support or improve metabolic dysfunction that occurs in alcohol‐induced liver disease. Future studies should also focus on the mechanism by which carnitine may improve cognitive function and metabolism in AUD.
Summary
This scoping review identified that carnitine levels are altered in AUDs and this may be related to the severity of disease and hepatic insufficiency. Studies primarily conducted in rodents and primary cell culture have shown that carnitine supplementation may support the clearance of toxic byproducts of EtOH metabolism (acetate, fatty acid ethyl esters, reactive oxygen species), support oxidative metabolism, reduce the deleterious effects of ROS and inflammation in AUD, and support mitochondrial function and neurotransmitter synthesis in the brain. All these effects would be beneficial to support the treatment of AUD. Ultimately, this underscores the need for further studies to better understand the underlying causes of changes in carnitine metabolism in AUDs, evaluate the effectiveness of carnitine supplementation in AUD, and understand the mechanisms by which carnitine may support treatment of AUDs.
Funding
This project was not funded.
Author Contribution
ABB, JGS, and KW were responsible for study concept and design. ABB and JGS performed the abstract and manuscript screening. Data extraction was completed by ABB, JGS, and ADB. ABB drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content and approved the final version for publication.
Conflict of Interest
The authors have nothing to disclose.
Supporting information
Table S1. Summary of studies evaluating plasma and tissue carnitine in AUD.
Table S2. Additional details for studies evaluating plasma and tissue carnitine in AUD.
Table S3. Summary of placebo‐controlled trials.
Data S1. Database. Web of Science..
Acknowledgments
The authors would like to acknowledge the Librarian A. Sascha Davis at The Ottawa Hospital for conducting the initial search.
References
- Adamo S, Siliprandi N, Lisa F, Carrara M, Azzurro M, Sartori G, Vita G, Ghidini O (1988) Effect of L‐Carnitine on ethanol and acetate plasma levels after oral administration of ethanol in humans. Alcohol Clin Exp Res 12:653–654. [DOI] [PubMed] [Google Scholar]
- Allenby C, Falcone M (2017) Using genetics to improve addiction treatment outcomes. Curr Behav Neurosci Reports 4:1–9. [Google Scholar]
- Alonso De La Pena C, Rozas I, Alvarez‐Prechous A, Pardinas MC, Paz JM, Rodriguez‐Segade S (1990) Free carnitine and acylcarnitine levels in sera of alcoholics. Biochem Med Metab Biol 44:77–83. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association, Arlington, VA. [Google Scholar]
- Amodio P, Angeli P, Merkel C, Menon F, Gatta A (1990) Plasma carnitine levels in liver cirrhosis: relationship with nutritional status and liver damage. J Clin Chem Clin Biochem 28:619–626. [DOI] [PubMed] [Google Scholar]
- Arafa HMM, Sayed‐Ahmed MM (2003) Protective role of carnitine esters against alcohol‐induced gastric lesions in rats. Pharmacol Res 48:285–290. [DOI] [PubMed] [Google Scholar]
- Ascha M, Wang Z, Ascha MS, Dweik R, Zein NN, Grove D, Brown JM, Marshall S, Lopez R, Hanouneh IA (2016) Metabolomics studies identify novel diagnostic and prognostic indicators in patients with alcoholic hepatitis. World J Hepatol 8:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustyniak A, Skrzydlewska E (2009) L‐Carnitine in the lipid and protein protection against ethanol‐induced oxidative stress. Alcohol 43:217–223. [DOI] [PubMed] [Google Scholar]
- Augustyniak A, Skrzydlewska E (2010) The influence of L‐carnitine suplementation on the antioxidative abilities of serum and the central nervous system of ethanol‐induced rats. Metab Brain Dis 25:381–389. [DOI] [PubMed] [Google Scholar]
- Best CA, Laposata M (2003) Fatty acid ethyl esters: Toxic non‐oxidative metabolites of ethanol and markers of ethanol intake. Front Biosci 8:e202–217. [DOI] [PubMed] [Google Scholar]
- Brass E (2003) Impact on carnitine homeostasis of short‐term treatment with the pivalate prodrug cefditoren pivoxil. Clin Pharmacol Ther 73:338–347. [DOI] [PubMed] [Google Scholar]
- Bykov IL, Belkovets AV (2004) Substantiation of the use of L‐carnitine for the treatment of alcoholism. Eksp Klin Farmakol 67:51–55. [PubMed] [Google Scholar]
- Bykov I, Jarvelainen H, Lindros K (2003) L‐Carnitine alleviates alcohol‐induced liver damage in rats: role of tumour necrosis factor‐alpha. Alcohol Alcohol 38:400–406. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Rizza V (1999) Effects of L‐carnitine on the formation of fatty acid ethyl esters in brain and peripheral organs after short‐term ethanol administration in rat. Neurochem Res 24:79–84. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Catalano C, Dinotta F, Bates TE, Calvani M, Stella AMG (2001) Effects of acetyl‐L‐carnitine on the formation of fatty acid ethyl esters in brain and peripheral organs after short‐term ethanol administration in rat. Neurochem Res 26:167–174. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI (2012) Alcohol metabolism. Clin Liver Dis 16:667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MB, Forte CA, Round JM (1987) Plasma carnitine and alcohol induced liver damage. Med Sci Res 15:671–673. [Google Scholar]
- Corbett R, Leonard BE (1984) Effects of carnitine on changes caused by chronic administration of alcohol. Neuropharmacology 23:269–271. [DOI] [PubMed] [Google Scholar]
- De Guzman Boado CA, Pandy JGP, Sarita MAT, Cua IHY (2019) Effect of L‐carnitine supplementation in non‐alcoholic fatty liver disease and glucose metabolism: a meta‐analysis. Hepatol Int 13:S210. [Google Scholar]
- de Sousa C, Leung NW, Chalmers RA, Peters TJ (1988) Free and total carnitine and acylcarnitine content of plasma, urine, liver and muscle of alcoholics. Clin Sci 75:437–440. [DOI] [PubMed] [Google Scholar]
- Deng X‐s, Deitrich R (2008) Putative role of brain acetaldehyde in ethanol addiction. Curr Drug Abuse Rev 1:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzyńska I, Szachowicz‐Petelska B, Skrzydlewska E, Figaszewski Z (2010) Effect of l‐carnitine on liver cell membranes in ethanol‐intoxicated rats. Chem Biol Interact 188:44–51. [DOI] [PubMed] [Google Scholar]
- Donohue T Jr (2007) Alcohol‐induced steatosis in liver cells. World J Gastroenterol 13:4974–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KM, Bird DA, al‐Salihi S, Hallaq Y, Cluette‐Brown JE, Goss KA, Laposata M (1994) Fatty acid ethyl esters are present in human serum after ethanol ingestion. J Lipid Res 35:428–437. [PubMed] [Google Scholar]
- Evans JD, Jacobs TF, Evans EW (2008) Role of acetyl‐L‐carnitine in the treatment of diabetic peripheral neuropathy. Ann Pharmacother 42:1686–1691. [DOI] [PubMed] [Google Scholar]
- Fuller RK, Hoppel CL (1983) Elevated plasma carnitine in hepatic cirrhosis. Hepatology 3:554–558. [DOI] [PubMed] [Google Scholar]
- Fuller RK, Hoppel CL (1988) Plasma carnitine in alcoholism. Alcohol Clin Exp Res 12:639–642. [DOI] [PubMed] [Google Scholar]
- Glass IB, Chalmers R, Bartlett S, Littleton J (1989) Increased plasma carnitine in severe alcohol dependence. Br J Addict 84:689–693. [DOI] [PubMed] [Google Scholar]
- Gülçin I (2006) Antioxidant and antiradical activities of L‐carnitine. Life Sci 78:803–811. [DOI] [PubMed] [Google Scholar]
- Haorah J, Rump TJ, Szlachetka A (2009) Mechanism of alcohol‐induced mitochondrial dysfunction in the brain: implication for neurodegeneration. Free Radic Biol Med 47:S111. [Google Scholar]
- Haorah J, Rump TJ, Xiong HG (2013) Reduction of brain mitochondrial beta‐oxidation impairs complex I and V in chronic alcohol intake: the underlying mechanism for neurodegeneration. PLoS One 8:e70833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper P, Schmidt D, Hultcrantz R, Cederblad G (1993) Liver carnitine content in chronic alcoholics in alcohol withdrawal. Eur J Gastroenterol Hepatol 5:177–181. [Google Scholar]
- Herz A (1997) Endogenous opioid systems and alcohol addiction. Psychopharmacology 129:99–111. [DOI] [PubMed] [Google Scholar]
- Hudson SA, Tabet N (2003) Acetyl‐l‐carnitine for dementia. Cochrane Database Syst Rev 2003:CD003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC (2014) Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta‐analysis. JAMA 311:1889. [DOI] [PubMed] [Google Scholar]
- Jones JD, Comer SD, Kranzler HR (2015) The pharmacogenetics of alcohol use disorder. Alcohol Clin Exp Res 39:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk NJ, Lingford‐Hughes AR (2014) The clinical pharmacology of acamprosate. Br J Clin Pharmacol 77:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kępka A, Chojnowska S, Śnitko R, Zwierz K, Waszkiewicz N (2016) Renal carnitine excretion following abstinence after chronic drinking. Adv Med Sci 61:160–163. [DOI] [PubMed] [Google Scholar]
- Kępka A, Waszkiewicz N, Zalewska‐Szajda B, Chojnowska S, Pludowski P, Konarzewska E, Szulc A, Ladny JR, Zwierz K, Szajda SD (2013) Plasma carnitine concentrations after chronic alcohol intoxication. Postepy Hig Med Dosw 67:548–552. [DOI] [PubMed] [Google Scholar]
- Kępka A, Zwierz P, Chojnowska S, Ochocińska A, Skorupa E, Szczepański M, Szajda SD, Waszkiewicz N (2019) Relation of plasma carnitine and aminotransferases to alcohol dose and time of dependence. Alcohol 81:62–69. [DOI] [PubMed] [Google Scholar]
- Khalil H, Peters M, Godfrey CM, Mcinerney P, Soares CB, Parker D (2016) An evidence‐based approach to scoping reviews. Worldviews Evidence‐Based Nurs 13:118–123. [DOI] [PubMed] [Google Scholar]
- Koob GF (2011) Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13:3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krähenbühl S, Reichen J (1997) Carnitine metabolism in patients with chronic liver disease. Hepatology 25:148–153. [DOI] [PubMed] [Google Scholar]
- Labib M, Abdel‐Kader M, Ranganath L, Martin S, Marks V (1989) Impaired renal tubular function in chronic alcoholics. J R Soc Med 82:139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposata EA, Lange LG (1986) Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science (80‐) 231:497–499. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Lin JS, Lin YC, Lin PT (2014) Effects of L‐carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: a randomized, placebo‐controlled trial. Nutr J 13:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo N, Frigeni M, Pasquali M (2016) Carnitine transport and fatty acid oxidation. Biochim Biophys Acta ‐ Mol Cell Res 1863:2422–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano N, Clementi G, Costantino G, Calvani M, Matera M (2000) Effect of acetyl‐L‐carnitine on ethanol consumption and alcohol abstinence syndrome in rats. Drugs Exp Clin Res 26:7–12. [PubMed] [Google Scholar]
- Marchesini G, Zoli M, Angiolini A, Dondi C, Bianchi FB, Pisi E (1981) Muscle protein breakdown in liver cirrhosis and the role of altered carbohydrate metabolism. Hepatology 1:294–299. [DOI] [PubMed] [Google Scholar]
- Martí‐Carvajal AJ, Gluud C, Arevalo‐Rodriguez I, Martí‐Amarista CE (2019) Acetyl‐L‐carnitine for patients with hepatic encephalopathy. Cochrane Database Syst Rev 1:CD011451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Andreoli S, Reina D, Di Nicola M, Ortolani I, Tedeschi D, Fanella F, Pozzi G, Iannoni E, D’Iddio S, Prof LJ (2011) Acetyl‐l‐Carnitine in the treatment of anhedonia, melancholic and negative symptoms in alcohol dependent subjects. Prog Neuro‐Psychopharmacology Biol Psychiatry 35:953–958. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Reina D, di Nicola M, Andreoli S, Tedeschi D, Ortolani I, Pozzi G, Iannoni E, D’Iddio S, Janiri L (2010) Acetyl‐L‐carnitine for alcohol craving and relapse prevention in anhedonic alcoholics: a randomized, double‐blind, placebo‐controlled pilot trial. Alcohol Alcohol 45:449–455. [DOI] [PubMed] [Google Scholar]
- Murad CA, Begg SJ, Griffiths PJ, Littleton JM (1977) Hepatic triglyceride accumulation and the ethanol physical withdrawal syndrome in mice. Br J Exp Pathol 58:606–615. [PMC free article] [PubMed] [Google Scholar]
- Nałȩcz KA, Miecz D, Berezowski V, Cecchelli R (2004) Carnitine: transport and physiological functions in the brain. Mol Aspects Med 25:551–567. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Lingford‐Hughes A, Erritzoe D, Stokes PRA (2015) The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci 16:305–312. [DOI] [PubMed] [Google Scholar]
- Osna NA, Donohue TM, Kharbanda KK (2017) Alcoholic liver disease: pathogenesis and current management. Alcohol Res 38:147–161. [PMC free article] [PubMed] [Google Scholar]
- Peterson J, Pearce PF, Ferguson LA, Langford CA (2017) Understanding scoping reviews. J Am Assoc Nurse Pract 29:12–16. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Klunk WE, Panchalingam K, Kanfer JN, McClure RJ (1995) Clinical and neurochemical effects of acetyl‐L‐carnitine in Alzheimer’s disease. Neurobiol Aging 16:1–4. [DOI] [PubMed] [Google Scholar]
- Reus VI, Fochtmann LJ, Bukstein O, Eyler AE, Hilty DM, Horvitz‐Lennon M, Mahoney J, Pasic J, Weaver M, Wills CD, McIntyre J, Kidd J, Yager J, Hong S‐H (2018) The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry 175:86–90. [DOI] [PubMed] [Google Scholar]
- Ribas GS, Vargas CR, Wajner M (2014) L‐carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 533:469–476. [DOI] [PubMed] [Google Scholar]
- Rudman D, Sewell CW, Ansley JD (1977) Deficiency of carnitine in cachectic cirrhotic patients. J Clin Invest 60:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachan DS, Rhew TH (1983) Lipotropic effect of carnitine on alcohol‐induced hepatic steatosis. Nutr Rep Int 27:1221–1226. [Google Scholar]
- Sachan DS, Rhew TH, Ruark RA (1984) Ameliorating effects of carnitine and its precursors on alcohol‐induced fatty liver. Am J Clin Nutr 39:738–744. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Ravagna A, Bella R, Colombrita C, Pennisi G, Calvani M, Alkon D, Calabrese V (2002) Long‐term ethanol administration enhances age‐dependent modulation of redox state in brain and peripheral organs of rat: protection by acetyl carnitine. Int J Tissue React 24:89–96. [PubMed] [Google Scholar]
- Sener G, Paskaloğlu K, Satiroglu H, Alican I, Kaçmaz A, Sakarcan A (2004) L‐carnitine ameliorates oxidative damage due to chronic renal failure in rats. J Cardiovasc Pharmacol 43:698–705. [DOI] [PubMed] [Google Scholar]
- Shen WW (2018) Anticraving therapy for alcohol use disorder: A clinical review. Neuropsychopharmacol Reports 38:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima AAF, Calvani M, Mehra M, Amato A (2005) Acetyl‐L‐carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo‐controlled trials. Diabetes Care 28:89–94. [DOI] [PubMed] [Google Scholar]
- Skinner MD, Lahmek P, Pham H, Aubin HJ (2014) Disulfiram efficacy in the treatment of alcohol dependence: a meta‐analysis. PLoS One 9:e87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiber A, Kerner J, Hoppel CL (2004) Carnitine: A nutritional, biosynthetic, and functional perspective. Mol Aspects Med 25:455–473. [DOI] [PubMed] [Google Scholar]
- Steiner JL, Lang CH (2015) Dysregulation of skeletal muscle protein metabolism by alcohol. Am J Physiol ‐ Endocrinol Metab 308:E699–E712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K (2016) Reevaluation of serum carnitine status in patients with liver cirrhosis. J Liver Res Disord Ther 2:58–64. [Google Scholar]
- Tempesta E, Troncon R, Janiri L, Colusso L, Riscica P, Saraceni G, Gesmundo E, Calvani M, Benedetti N, Pola P (1990) Role of acetyl‐L‐carnitine in the treatment of cognitive deficit in chronic alcoholism. Int J Clin Pharmacol Res 10:101–107. [PubMed] [Google Scholar]
- Veronese N, Stubbs B, Solmi M, Ajnakina O, Carvalho AF, Maggi S (2018) Acetyl‐l‐carnitine supplementation and the treatment of depressive symptoms: a systematic review and meta‐analysis. Psychosom Med 80:154–159. [DOI] [PubMed] [Google Scholar]
- Wawrzeńczyk A, Nałȩcz KA, Nałȩcz MJ (1995) Effect of externally added carnitine on the synthesis of acetylcholine in rat cerebral cortex cells. Neurochem Int 26:635–641. [DOI] [PubMed] [Google Scholar]
- World Health Organization, Alcohol, Drugs and Behaviors (2018) Vladimir P, Dag R (Eds.), Global status report on alcohol and health 2018, 450. [Google Scholar]
- Zindel LR, Kranzler HR (2014) Pharmacotherapy of alcohol use disorders: seventy‐five years of progress. J Stud Alcohol Drugs Suppl 75(Suppl 1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of studies evaluating plasma and tissue carnitine in AUD.
Table S2. Additional details for studies evaluating plasma and tissue carnitine in AUD.
Table S3. Summary of placebo‐controlled trials.
Data S1. Database. Web of Science..


