Abstract
The COVID-19 pandemic has resulted in widespread use of complementary and alternative medicines. Tinospora cordifolia is a widely grown shrub which has been commonly used in India’s traditional system of Ayurveda for its immune booster properties and has been extensively used as prophylaxis against COVID-19. Six patients (4 women, 2 men) with a median (IQR) age of 55 years (45–56) and with an history of Tinospora cordifolia consumption presented with symptoms of acute hepatitis during the study period of 4 months in the COVID-19 pandemic. The median (IQR) duration of Tinospora cordifolia consumption was 90 days (21–210). The median (IQR) peak bilirubin and AST were 17.5 mg/dl (12.2–24.9) and 1350 IU/ml (1099-1773), respectively. The patients had either a definite (n = 4) or probable (n = 2) revised autoimmune hepatitis score with an autoimmune pattern of drug-induced liver injury on biopsy. Four of these patients (all women) had underlying silent chronic liver disease of possible autoimmune etiology associated with other autoimmune diseases – hypothyroidism and type 2 diabetes mellitus. One of the three patients treated with steroids decompensated on steroid tapering. The other five patients had resolution of symptoms, liver profile, and autoimmune serological markers on drug withdrawal/continuing steroid treatment. The median (IQR) time to resolution from discontinuing the herb was 86.5 days (53-111). Tinospora cordifolia consumption seems to induce an autoimmune-like hepatitis or unmask an underlying autoimmune chronic liver disease, which may support its immune stimulant mechanism. However, the same mechanism can cause significant liver toxicity, and we recommend that caution be exercised in the use of this herb, especially in those predisposed to autoimmune disorders. Besides, in patients presenting with acute hepatitis, even in the presence of autoimmune markers, a detailed complementary and alternative medicine history needs to be elicited.
Keywords: tinospora cordifolia, Giloy, drug induced liver injury, drug-induced autoimmune hepatitis, herb induced liver injury
Abbreviations: AIH, Autoimmune hepatitis; ALT, Alanine Transaminase; ASMA, Anti-Smooth Muscle Antibody; AST, Aspartate Transaminase; CAM, Complementary and Alternative Medicines; COVID-19, Coronavirus Disease of 2019; DILI, Drug-Induced Liver Injury; IgG, Immunoglobulin G; IQR, Inter Quartile Range; LFT, Liver Function Tests; RUCAM, Roussel Uclaf Causality Assessment Method; SMT, Standard medical treatment; TC, Tinospora cordifolia; ULN, Upper Limit of Normal; USG, Ultrasonography
The outbreak of COVID-19 in December 2019 has claimed more than 3.7 million lives worldwide till now.1 The search for agents to mitigate the ill effects of COVID-19 infection has led to the use of prescription agents including investigational agents as well as an increased use of complementary and alternative medicines (CAMs).2
CAM drugs are available over-the-counter from chemists without a doctor’s prescription. Although they may have benefits, they also have a reputation for lesser side effects compared to modern medicine. However, there have been several documented cases of drug-induced liver injury (DILI)3, 4, 5 due to CAM drugs. Also, more may be implicated. Their toxicity may arise from direct and indirect mechanisms through the metabolites of the herb or their interactions with other drugs including contaminants.3 We describe what is to our knowledge the first histologically documented experience of six patients with a drug-induced autoimmune-like hepatitis secondary to consumption of Tinospora cordifolia (TC), an Indian herb popularly used for its “immunity booster” effects during the pandemic. TC is known by different names in the various Indian languges6 (Sanskrit: Guduchi, Amrita; Hindi: Giloy, Guranch; Malayalam: Amrytu; Punjabi: Batindu; Tamil: Amrida Valli; Marathi: Gulavela, Ambarvel; Bengali: Giloe; Gujarati: Gado; Telugu: Tippa teega; Oriya: Gulochi, Gulanch; Kannada: Arnryta balli; Sikkim: Gurjo).
Methods
In this hospital-based observational study, we observed 6 patients who presented with herbal-induced liver injury, during September 2020 to December 2020 and followed up until March 2021. The study was cleared by the Institutional Ethics Committee (EC/1089/2021) and informed consent was obtained from the study participants.
We included outpatients and inpatients aged >18 years with a presentation of acute hepatitis and a recent exposure to ingestion/consumption of a distinctive formulation containing TC. We excluded patients with significant alcohol consumption (more than 40 g of alcohol per day for men, and more than 20 g of alcohol per day for women, both more than 10 years duration.)7
We also excluded acute viral hepatitis A, B, C and E by serological tests and Wilson disease.
The demographic details, clinical, biochemical (complete blood count, liver function test [LFT] and international normalised ratio) and autoimmune markers (anti-nuclear antibody [ANA], anti-smooth muscle antibody [ASMA] and serum immunoglobulin G (IgG)] were recorded. All patients underwent ultrasonography (USG) of the abdomen. Patients underwent a liver biopsy to assess severity of liver injury and determining the likely aetiology. The presentation and peak values of bilirubin and liver enzymes are depicted in the table. Patients were followed up in the hospital until total bilirubin levels were less than 5 mg/dl, and aspartate transaminase (AST) and alanine transaminase (ALT) were less than 2 times upper limit of normal along with resolution of symptoms. In patients with positive autoimmune markers or raised IgG, the specific abnormal test was repeated on resolution of symptoms. Revised AIH (autoimmune hepatitis) scores pre- and post-treatment in those who received steroids and pre-treatment in those who did not were calculated.8 The updated RUCAM (Roussel Uclaf Causality Assessment Method) score9 was calculated for the likelihood for drug causality excluding re-challenge. A brief description of patients’ profile is summarised in Table 1.
Table 1.
Biochemical, Serological and Histopathological Profile of Six Patients Consuming Tinospora Cordifolia.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | ||
|---|---|---|---|---|---|---|---|
| Age/sex | 40/M | 54/F | 38/M | 62/F | 56/F | 56/F | |
| Laboratory parameters | Hb (g/dl) | 13.7 | 10.9 | 9 | 12.4 | 11.5 | 11.8 |
| TLC (cells/cmm) | 16000 | 6200 | 9200 | 8400 | 8550 | 6690 | |
| Platelets (cells/cmm) | 388000 | 187000 | 371000 | 103000 | 241000 | 140000 | |
| Presentation total bilirubin (direct bilirubin) (mg/dl) | 7.9 (6.7) | 15.3 (10.9) | 7.4 (3.9) | 9.1 (5.7) | 12.2 (7.5) | 9.13 (4.41) | |
| Peak total bilirubin (direct bilirubin) (mg/dl) | 45.1 (25) | 24.9 (21) | 20 (10.7) | 15.1 (12.5) | 12.2 (7.5) | 9.1 (4.4) | |
| On presentation AST (IU/L) | 1773 | 1195 | 760 | 369 | 1099 | 347 | |
| Peak AST (IU/L) | 1773 | 1195 | 1504 | 2222 | 1099 | 455 | |
| On presentation ALT (IU/L) | 2894 | 768 | 560 | 202 | 256 | 207 | |
| Peak ALT (IU/L) | 3114 | 768 | 1482 | 855 | 256 | 472 | |
| Time to normalisation of LFT (in days) | 95 | 164 | 78 | 111 | 38 | 53 | |
| Autoimmune profile (pre/post)a | ANA | Negative/- | 1:100/Negative | 1:100/-ve | 1:320/1:320 | Negative/- | Negative/- |
| Anti-SMA | Negative/- | Negative/- | – | Positive/Negative | Negative/- | Weakly positive/Negative | |
| Serum IgG (700–1600 mg/dl) | Normal/- | Normal/- | Normal/- | – | 2570/1721 | 2045/1680 | |
| Revised AIH scoreb(pre/post when steroids given) | 12 | 19/21 | 15 | 19/22 | 18/21 | 18 | |
| Liver biopsy | Interface hepatitis | ++ | +++ | + | +++ | ++ | + |
| Lymphocytes | ++ | ++ | ++ | ++ | ++ | ++ | |
| Eosinophils | + | ++ | +++ | +++ | ++ | ++ | |
| Plasma cells | + | + | + | ++ | ++ | ++ | |
| Neutrophils | – | ++ | – | +++ | – | – | |
| Pattern | Hepatocellular | Hepatocellular + Cholestatic | Hepatocellular | Hepatocellular | Hepatocellular | Hepatocellular | |
| Updated RUCAM score refc c(without rechallenge) | 4 | 5 | 4 | 7 | 7 | 4 | |
Abbreviations - M: Male, F: Female, Hb: Haemoglobin, TLC: Total leucocyte count, AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, LFT: Liver function tests, ANA: Anti-nuclear antibody, Anti-SMA: Anti-smooth muscle antibody, IgG: Immunoglobulin G, AIH: Autoimmune hepatitis.
+++: Prominent/Abundant ++: Moderate +: Mild/Occasional -: Not seen/done.
Pre-stopping the drug and post-stopping the drug, - implies not done as previous ANA/ASMA were negative or IgG was normal.
Revised AIH score: Pre-treatment: Definite AIH >15 Probable AIH l0-15 Post-treatment: Definite AIH >17 12 Probable AIH 12–17.
Updated RUCAM score and resulting causality grading: ≤0, excluded; 1–2, unlikely; 3–5, possible; 6–8, probable; and ≥9 highly probable.
Case 1
A previously healthy 40-year-old male without co-morbidities, presented with jaundice of 15 days duration. On persistent probing, he gave a history of consumption of TC plant twigs (10–12 pieces) boiled with cinnamon and cloves in half a glass of water, once in 2 days for 3 months prior to presentation. USG of the abdomen was unremarkable. He underwent a percutaneous liver biopsy which showed features of hepatocellular pattern of liver injury—with lymphoplasmacytic cell infiltrate, interface hepatitis and foci of necrosis—suggesting the diagnosis of DILI with autoimmune features. He was managed with standard medical treatment (SMT) which included multivitamins and ondansetron for associated nausea. He was followed up for 5 months till complete resolution of symptoms and normalisation of liver function.
Case 2
A 54-year-old female, with type 2 diabetes mellitus, presented with jaundice since 1 week. A 7-month history of unsupervised consumption of TC plant (1 twig per day), which was boiled and extract consumed, was obtained. Evaluation for cause revealed a positive ANA (1:100), negative ASMA, negative viral markers and normal IgG. USG features showed a liver with coarse echotexture, spleen of 13.4 cm and minimal free fluid in the abdomen. A percutaneous liver biopsy showed mixed patterns of liver injury (hepatocellular and cholestatic) with features of lymphocytic, neutrophilic and eosinophilic infiltrate, prominent interface hepatitis, intracytoplasmic and canalicular cholestasis and altered architecture (Figure 1). She was managed with SMT. In view of chronicity, she was started on oral prednisolone in a dose of 40 mg which was tapered over a period of 10 weeks following which there was resolution of her symptoms, improvement in LFTs and she was advised regular follow up.
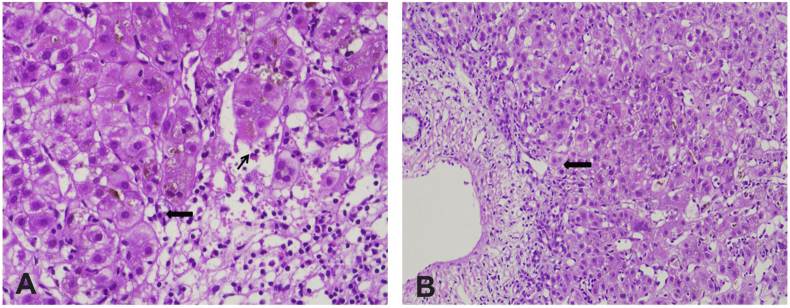
Figure 1.
A: Liver biopsy (haematoxylin and eosin, 400X) showing areas of necrosis with lymphocytic infiltrate admixed with eosinophils (thin black arrow) and plasma cells (thick black arrow). B: Liver biopsy (haematoxylin and eosin, 400X) shows interface hepatitis (thick black arrow).
Case 3
A 38-year male with beta-thalassemia minor presented with jaundice of 1-week duration. He gave a history of consumption of 3–4 TC plant twigs—boiled and extract consumed 15 ml/day—for 6 months prior to presentation. Work up for the aetiology showed a positive ANA (1:100). USG showed hepatomegaly (16 cm) with diffuse fatty infiltration and splenomegaly (17.3 cm). A percutaneous liver biopsy suggested the diagnosis of drug-induced hepatitis with hepatocellular pattern of liver injury along with moderate lymphocytic infiltrate admixed with plenty of eosinophils, few plasma cells and mild interface hepatitis. He was managed with SMT and followed up until complete resolution of symptoms and LFTs.
Case 4
A 62-year-old female with type 2 diabetes mellitus presented with complaints of malaise, reduced appetite and yellowish discolouration of urine, eyes and skin with abdominal distension since 15 days. She confirmed consumption of commercially available syrup containing TC plant—15 ml/day, every alternate day for a month, prior to the onset of her symptoms.
Investigations revealed a positive ANA (1:320) and ASMA. Imaging showed hepatomegaly and ascites. A trans-jugular liver biopsy suggested a diagnosis of AIH suggested by lymphoplasmacytic infiltrate with eosinophils and neutrophils, as well as interface hepatitis. There was also cirrhosis suggested by marked lobular disarray, pseudoglandular transformation and bridging hepatic fibrosis. She was treated with standard medical therapy including low salt diet and diuretics for ascites and started on oral prednisolone 40 mg per day. She initially showed clinical improvement and improving trend of LFTs. However, on tapering of steroids, she came back with increasing ascites and oliguria and succumbed to hepatorenal syndrome around 120 days from the first presentation.
Case 5
A 56-year-old female with hypothyroidism presented with yellowish discolouration of urine and eyes. A short, 3-week history of consumption of TC plant—boiled extract of 1 twig, 2–3 days/week—was obtained. Standard investigations for aetiology were negative except for a high serum IgG of 2570 mg/dl. The autoimmune markers were negative. USG showed mild ascites, nodular liver and spleen of 12.3 cm. A transjugular liver biopsy showed lymphoplasmacytic infiltrate admixed with plasma cells and eosinophils, moderate interface hepatitis, fibrosis and altered architecture suggestive of an autoimmune cirrhosis. SMT and tapering doses of prednisolone starting with 40 mg orally over 6 weeks led to resolution of symptoms with improvement in LFT. She was continued on maintenance dose of steroids and advised close follow up.
Case 6
A 56-year-old female with hypothyroidism presented with jaundice of 20 days duration. History of TC plant formulation in the form of commercially available tablets—1 pill a day, for 3 months prior to presentation—was obtained. Routine evaluation for cause of liver injury showed a weakly positive ASMA and a high serum IgG (2045 mg/dl). ANA was negative. USG showed diffuse heterogeneous echotexture of liver and normal sized spleen. A percutaneous liver biopsy showed chronic hepatitis with lymphoplasmacytic infiltrate and interface hepatitis with significant bridging fibrosis suggesting the possibility of AIH. She was managed with SMT, leading to complete symptomatic and biochemical resolution. There was no relapse of hepatitis after stopping TC and a follow up of 2 months.
Results
Of 6 patients in our case series, 4 (all women) had features of chronic liver disease with autoimmune features on liver biopsy (patients 2, 4, 5 and 6). All four also had associated autoimmune diseasestwo had diabetes mellitus and two had hypothyroidism. Three of the four had positive autoimmune markers and 1 had significantly high IgG of 2570 mg/dl (normal up to 1600 mg/dl). Of the 2 patients without evidence of chronicity, one had ANA positivity (patient 3) and the other had interface hepatitis with plasma cells and eosinophils (patient 1).
Four of the six patients consumed TC plant in the form of boiled extracts of its twigs, while one patient consumed it in the commercially available tablet formulation and another one consumed in the form of commercially available syrup. The median interquartile range (IQR) duration of TC consumption was 90 days (21–210). All of them had self-prescribed the TC formulation.
All patients discontinued consumption of TC, which was advised at presentation. None of the patients had any features of hypersensitivity like rash, fever or eosinophilia. The demographic details, LFTs on presentation and peak values, details of autoimmune serology, revised AIH score, the updated RUCAM score, autoimmune features on biopsy and duration of consumption of TC and of the illness have been described in the table. The revised AIH score was suggestive of definite AIH pre- and post-treatment in all 3 patients who received steroids and in 1 patient who did not receive steroids. Two patients who did not receive steroids had scores in the probable range.
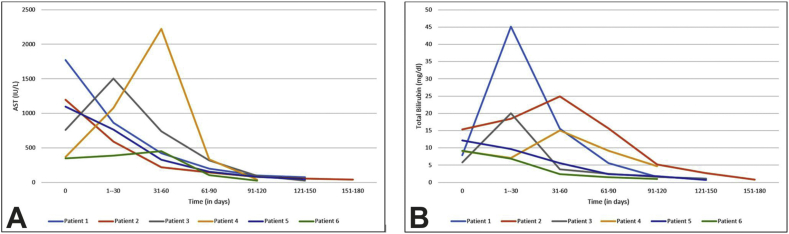
The updated RUCAM score showed possible DILI in 4 and probable DILI in 2 patients. The median(IQR) duration of resolution of the LFTs in our patients was 86.5 days (53–111). The median (IQR) peak AST was 1350 IU/L (1099–1773) and median (IQR) peak ALT level was 812 IU/L (472–1482). In all patients, AST was higher than ALT. The median (IQR) peak total bilirubin level was 17.5 mg/dl (12.2–24.9). Figure 2A and B shows trend of peak AST and bilirubin respectively. The alkaline phosphatase rise was minimal in all patients and none of the patients had any coagulopathy. Of the four patients with evidence of chronic liver disease, 3 received steroids and in one patient the liver profile rapidly improved after stopping TC. Of the three, one patient decompensated after reducing steroids, one continued to do well on discontinuation and follow up and the third patient was continued on steroids as the AST/ALT values came down slowly.
Figure 2.
A: Trend of AST (aspartate transaminase) values over time in the six patients with a history of Tinospora cordifolia consumption. B: Trend of total bilirubin values over time in the six patients with a history of Tinospora cordifolia consumption.
Discussion
TC is the scientific name for a commonly used Indian plant in Ayurveda, a traditional system of medicine. It is described as heart-leaved moonseed in the English language (from the shape of its leaves) (Figure 3). It is a widely grown glabrous, deciduous climbing shrub which has been described in traditional medicine texts to have a long list of health benefits.10 Preparations utilise the stem and root of the plant which is consumed in the form of capsules, powder or juice or in an unprocessed form. Its described benefits have included anti-inflammatory, anti-pyretic properties, anti-viral and anti-cancer, especially immune boosting properties, which made it popular during the pandemic.10, 11, 12 Rege et al have described an immune protective mechanism with oral administration of TC in improving surgical outcome of patients with obstructive jaundice.13 Barring one isolated case of acute hepatocellular injury due to the use of TC,14 there have been no reports of TC-related liver injury. Liver biopsy was not performed in the reported case. Although updated RUCAM scores to determine the causality were in the possible-probable range for DILI in our patients, these did not include re-challenge which would have possibly increased the scores. In view of severe injury and the fact that the patients improved on TC withdrawal, we did not feel it was ethical to re-challenge. Besides, RUCAM has not been found to be reliable in the presence of chronic liver disease.15 A limitation of our study was not having the serologies for Epstein–Barr virus, herpes simplex virus and cytomegalovirus which could have further modified the scores. We also did not analyse the contents of the plant parts which the patients had consumed.
Figure 3.
Branches and heart-shaped leaves of the plant Tinospora cordifolia brought by a patient.
Although the mechanisms of liver injury due to CAMs could be varied,3 we propose that the liver injury seen in our patients could be because of an autoimmune-like hepatitis due to consumption of TC, or the unmasking of latent chronic autoimmune liver disease. This mechanism was supported by the fact that majority had a revised AIH score in the definite range in spite of not including other antibodies in the panel, which was another limitation of our study. Most drug-induced autoimmune liver injuries are an acute idiosyncratic reaction,16 which was also supported by the fact that one patient takes the drug for only 3 weeks on alternate days.
All four patients with an underlying chronic liver disease were female and had an associated autoimmune disorder—two had hypothyroidism and two had type 2 diabetes mellitus. In two of these four patients, TC precipitated decompensation with development of ascites that needed a transjugular liver biopsy. Three of these patients with chronic liver disease received steroids (oral prednisolone) as their LFTs continued to deteriorate, whereas one showed spontaneous improvement after drug withdrawal. The mechanism for unmasking of latent chronic autoimmune liver disease may be explained by the immune-stimulant effects of TC,11 which could have exacerbated an underlying pre-existing heightened immune response to self-antigens expressed by the liver. The other two of the six patients -both males—seemed to have a de novo drug-induced AIH with one of them being seronegative but with high IgG levels as has been seen in this type of DILI.16
Drug metabolites of TC may provide the antigenic stimulus or the immune trigger. Most of the patients’ positive autoimmune serological markers became negative and serum IgG levels normalised on repeat testing at resolution except in one patient who succumbed to her illness. Thus, all our patients could be categorised as having “drug-induced autoimmune-like hepatitis””a term described by Czaja16 classically seen with the drugs minocycline and nitrofurantoin.16, 17, 18 TC has been described to increase IgG antibodies with macrophage activation and has been used in its immune boosting potential in HIV patients.19 Syringin, cordiol, cordioside and cordifoliosides A and B have been described to be the active ingredients responsible for its immune-stimulant activities.20,21
A short course of glucocorticoids as mentioned in literature and also in our experience may be used for management of cases of immune-mediated DILI.16 A relapse after steroid withdrawal may be seen in those with underlying autoimmune liver disease in 50%–87%,16 as was seen in patient 4 while patients with de novo drug-induced autoimmune injury usually do not relapse22 after drug withdrawal (patients 1 and 3). The four patients with an underlying liver disease will need to be followed up vigilantly in the long term for a relapse.
DILI secondary to TC plant extract ingestion should be an important differential diagnosis in patients presenting acutely with hepatocellular jaundice in the absence of a viral aetiology, especially in the COVID-19 pandemic, although the herb has been in use for decades. The medical profession needs to be familiar with the knowledge of the various local names of TC as the relevant information may be completely missed. We experienced this in our patients who primarily resided in Mumbai but originated from different parts of the country and identified TC with their own local name. Serological and histological evidence of autoimmunity needs to be investigated in patients presenting with an acute hepatitis but a negative viral serology. Presence of autoimmune markers may not only imply an autoimmune hepatitis but also suggest the diagnosis of drug-induced autoimmune-like hepatitis. Conversely, in any patient presenting with an AIH, efforts should be made to elicit a history of consuming a drug or CAM as this information is often not volunteered by patients on their own. The drug may have benefitted some through its immune-stimulant action given the fact that it seems to have triggered an autoimmune process. However, there is a need to urge caution and a warning about the potential to cause liver toxicity. Also, a recommendation to monitor LFTs especially in the high risk subset of patients (associated autoimmune disorders) needs to be highlighted.
Credit authorship contribution statement
Aabha Nagral: Conceptualisation, Methodology, Writing- Review and editing, Supervision, Kunal Adhyaru: Writing – original draft, Investigation, Omkar Rudra: Formal analysis, writing-review and editing, Amit Gharat: Writing- Review and Editing the manuscript, Sonal Bhandare: Methodology, Writing-review and editing.
Conflicts of interest
The authors have none to declare.
Acknowledgement
The authors thank Dr Harshad Devarbhavi for his inputs on the manuscript.
Funding
None.
References
- 1.https://covid19.who.int/.
- 2.Koshy J. The Hindu; New Delhi: October 18, 2020. Coronavirus | in Rush for ‘immunity’, Ayurveda Supplements Have an Untested Run. [Google Scholar]
- 3.Björnsson E.S. Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci. 2016;17:224–230. doi: 10.3390/ijms17020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philips C.A., Ahamed R., Rajesh S., George T., Mohanan M., Augustine P. Comprehensive review of hepatotoxicity associated with traditional Indian Ayurvedic herbs. World J Hepatol. 2020;12:574–595. doi: 10.4254/wjh.v12.i9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devarbhavi H., Aithal G., Treeprasertsuk S., et al. Asia pacific association of study of liver. Drug induced liver injury: asia pacific association of study of liver consensus guidelines. Hepatol Int. 2021 Feb 27 doi: 10.1007/s12072-021-10144-3. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.https://herbs.indianmedicinalplants.info/index.php/61-guduci-tinospora-cordifolia.
- 7.Osna N.A., Donohue T.M., Jr., Kharbanda K.K. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38:147–161. [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez F., Berg P.A., Bianchi F.B., et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 9.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17:14. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.ayush.gov.in/.
- 11.Kalikar M.V., Thawani V.R., Varadpande U.K., et al. Immunomodulatory effect of Tinospora cordifolia extract in human immuno-deficiency virus positive patients. Indian J Pharmacol. 2008;40:107–110. doi: 10.4103/0253-7613.42302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma V., Kaushik S., Pandit P., Dhull D., Yadav J.P., Kaushik S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl Microbiol Biotechnol. 2019;103:881–891. doi: 10.1007/s00253-018-9488-1. [DOI] [PubMed] [Google Scholar]
- 13.Rege N., Bapat R.D., Koti R., Desai N.K., Dahanukar S. Immunotherapy with Tinospora cordifolia: a new lead in the management of obstructive jaundice. Indian J Gastroenterol. 1993;12:5–8. [PubMed] [Google Scholar]
- 14.Karousatos C.M., Lee J.K., Braxton D.R., et al. Case series and review of Ayurvedic medication induced liver injury. BMC Complement Med Ther. 2021;21:91. doi: 10.1186/s12906-021-03251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teschke R., Danan G. Prospective Indian study of DILI with confirmed causality using the roussel Uclaf causality assessment method (RUCAM): a report of excellence. Ann Hepatol. 2017;16:324–325. doi: 10.5604/16652681.1235471. [DOI] [PubMed] [Google Scholar]
- 16.Czaja A.J. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56:958–976. doi: 10.1007/s10620-011-1611-4. [DOI] [PubMed] [Google Scholar]
- 17.Björnsson E., Talwalkar J., Treeprasertsuk S., et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatol Baltim Md. 2010;51:2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 18.Fontana R.J., Seeff L.B., Andrade R.J., et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatol Baltim Md. 2010;52:730–742. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chetan B., Nakum A. Use of natural compounds, chitin and tinosporin for the treatment of the targeted viruses (retroviruses) (HIV-1, HIV-2) all subgroups, HTLV and other viral disease. Indian patent App. 2010 IN 2010MU01350 A20100730. [Google Scholar]
- 20.Kapil A., Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J Ethnopharmacol. 1997;58:89–95. doi: 10.1016/s0378-8741(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 21.Atal C.K., Sharma M.L., Kaul A., Khajuria A. Immunomodulating agents of plant origin. I: preliminary screening. J Ethnopharmacol. 1986;18:133–141. doi: 10.1016/0378-8741(86)90025-5. [DOI] [PubMed] [Google Scholar]
- 22.van Gerven N.M.F., Verwer B.J., Witte B.I., et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58:141–147. doi: 10.1016/j.jhep.2012.09.009. [DOI] [PubMed] [Google Scholar]