Figure 4.
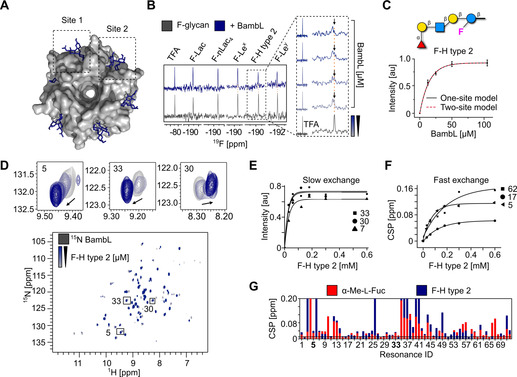
Bacterial lectin (BambL) binding to F‐glycans. A) Surface diagram of the crystal structure of BambL in complex with H‐F type 2 (PDB: 3zzv). Sites 1 and 2 correspond to the carbohydrate‐binding sites within a monomer and between two monomers, respectively. B) 19F NMR screening of F‐glycans alone (gray) and in presence of BambL (blue). BambL binds F‐Lex, F‐Ley, and F‐H type 2 strongly as shown by CSP in presence of protein (orange line). The 19F NMR titration spectra shows F‐H type 2 undergoing slow exchange on the chemical shift timescale upon increase of BambL concentration. C) The K d of F‐H type 2 was calculated from the changes in peak intensity and fitted to one‐ and two‐site models resulting in a K d of 9±2 μm. D) TROSY NMR verified F‐H type 2 binding to 15N‐labeled BambL. Given that BambL has two binding sites, peaks showing a slow (30, 7, and 33), intermediate and fast exchange (5, 17, and 62) on the chemical shift timescale have been observed upon titration of F‐H type 2. One‐site model for slow (E) and fast exchange (F) peaks was applied to derive the K d values of 12±8 μm and 94±33 μm, respectively. G) CSP plot showing the resonances perturbed in presence of α‐Me‐l‐fucose and F‐H type 2.
