Figure 4.
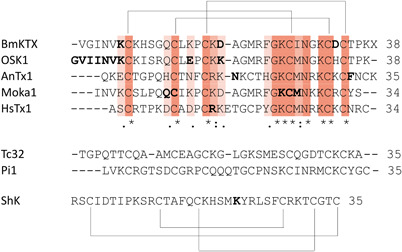
Above: the aligned sequences of BmKTX 101 (1BKT), OSK1 102 (1SCO), AnTx 103 (KAX6C_ANUPH), moka1 104 (2KIR), and HsTx1 105 (1QUZ) are shown together with the pattern of their disulfide bridges. Residues in bold are amino acids of which mutations have been shown to alter Kv1.3 binding. Middle: Sequences of Tc32 106 (2JP6) and Pi1 107 (1WZ5) are shown. Below: Sequence of ShK 108 (1ROO) is shown together with the pattern of its disulfide bridges. Residue in bold is an amino acid of which mutations have been shown to alter Kv1.3 binding [Color figure can be viewed at wileyonlinelibrary.com]
