Fig. 1.
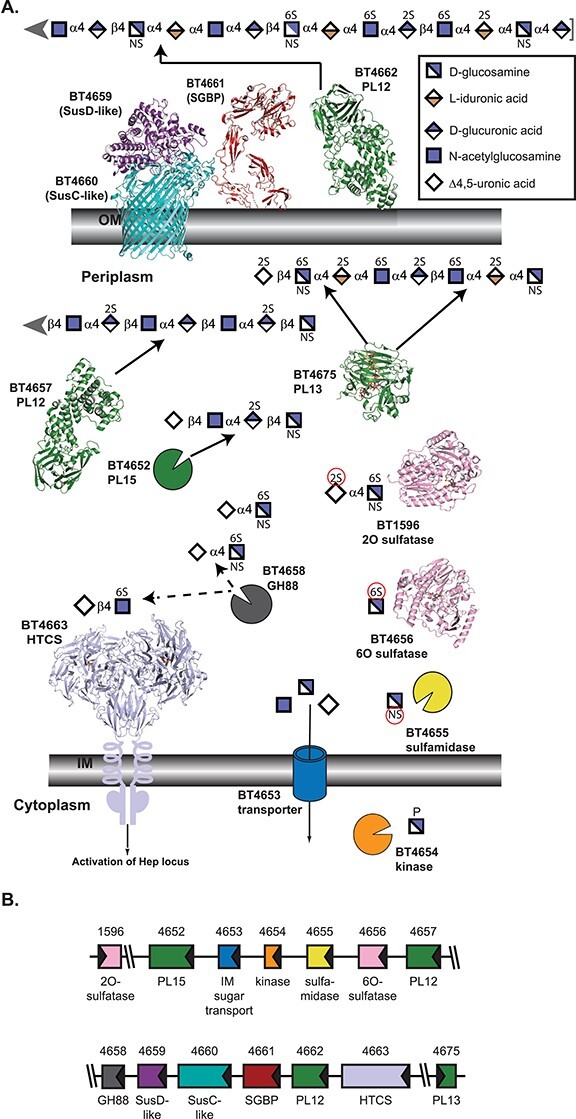
Architecture and activities within B. theta PULHEP, as described by Cartmell et al. 2017. (A) Cartoon representation of the extracellular and periplasmic components of PULHep. Heparin/heparan sulfate is captured at the cell surface, minimally processed by BT4662-PL12, and the oligosaccharides are imported by the SusCD-like complex with assistance from the SGBP BT4661. Once imported, an elaborate network of enzymes reduces the glycan to desulfated monosaccharides. The SusC-like protein is not the Hep SusC-like protein but is the recently determined homolog BT2264 (Glenwright et al. 2017) (PDB 4FQ8). The SusD-like protein is the Hep SusD-like protein (PDB 3IHV). The SGBP structure is a combination of the ligand (Δ4,5UA2S-GlcNS6S-IdoA2S-GlcN6S-IdoA2S-GlcNS6S) from PDB 4AK2 and the full-length protein from PDB 4AK1 (Cartmell et al. 2017). SGBP ligand and all subsequent ligands are colored in dark gray. Unliganded 4662-PL12 is PDB 4FNV (Dong et al. 2012). Unliganded 4657-PL12 is PDB 5JMF (Ulaganathan et al. 2017). 4675-PL13 is shown complexed with a heparin-derived dodecasaccharide and PDB 3INA (Han et al. 2009). The 2O-sulfatase BT1596-S1_9 is complexed with Δ4,5UA2S-GlcNS6S (PDB 5G2T (Cartmell et al. 2017)). The 6O-sulfatase BT4656-S1_11 is complexed with GlcNS6S (PDB 5G2V (Cartmell et al. 2017)). The dimeric periplasmic domain of the HTCS BT4663 is complexed with Δ4,5UA-GlcNAc6S (PDB 4A2M (Lowe et al. 2012)). (B) Architecture of the genetic loci. The gene representations are to scale and colored the same as their protein products.
