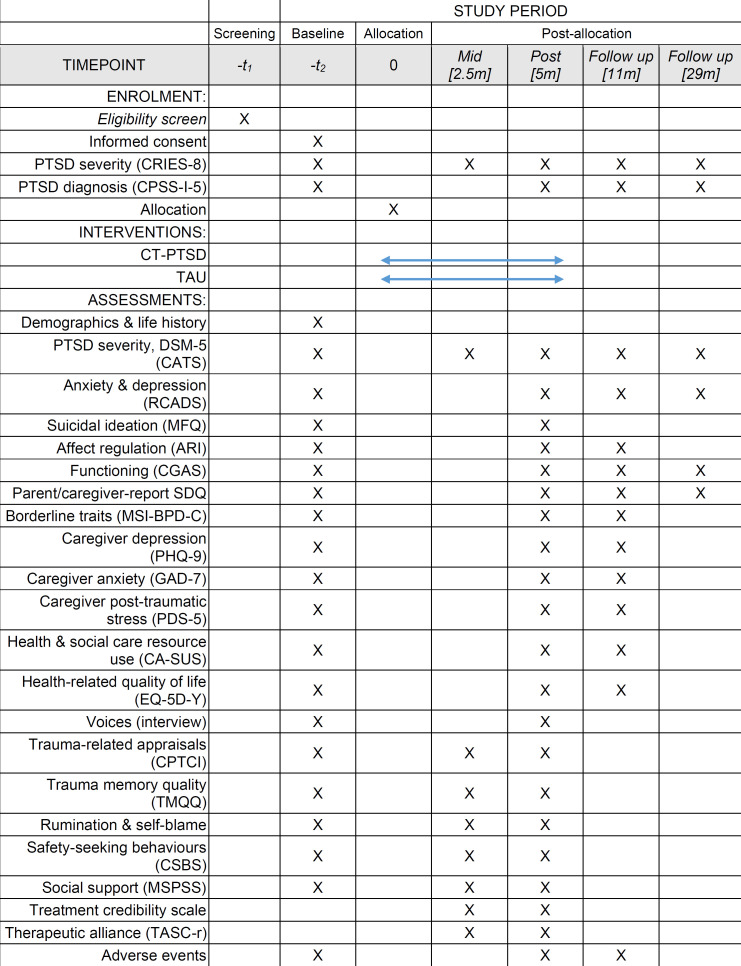
Figure 1.
Standard Protocol Items: Recommendations for Interventional Trials diagram detailing trial activities and measures and their timing. ARI, Affective Reactivity Index; CA-SUS, Child and Adolescent Service Use Schedule; CATS, Child and Adolescent Trauma Screen; CGAS, Children’s Global Assessment Scale; CPSS-I-5, Child PTSD Symptom Scale for DSM-5, interviewer version; CPTCI, Children’s Post-Traumatic Cognitions Inventory; CRIES-8, Child Revised Impact of Event Scale-8; CSBS, Child Safety Behaviour Scale; CT-PTSD, cognitive therapy for PTSD; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; EQ-5D-Y, EuroQol measure of health-related quality of life; GAD-7, Generalised Anxiety Disorder Assessment; MFQ, Mood and Feelings Questionnaire; MSI-BPD-C, McLean Screening Instrument for Borderline Personality Disorder, caregiver version; MSPSS, Multidimensional Scale of Perceived Social Support; PDS-5, Post-traumatic Stress Diagnostic Scale, DSM-5 version; PHQ-9, Patient Health Questionnaire; PTSD, post-traumatic stress disorder; RCADS, Revised Child Anxiety and Depression Scale; SDQ, Strengths and Difficulties Questionnaire; TASC-r, Therapeutic Alliance Scale for Children, revised; TAU, treatment as usual; TMQQ, Trauma Memory Quality Scale,