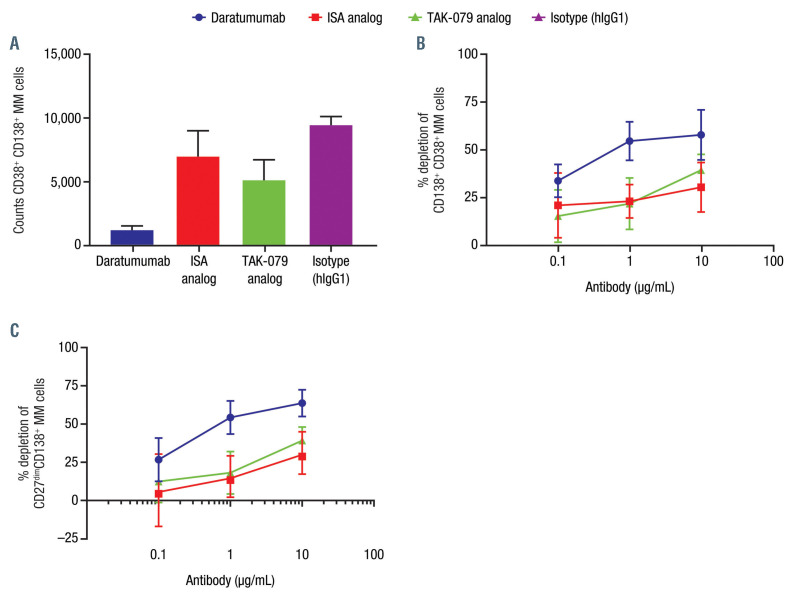
Figure 3.
Daratumumab depletes multiple myeloma cells in patient samples as depicted by (A) counts of CD38+CD138+, (B) percent depletions of CD138+CD38+, and (C) percent depletion of CD27dimCD138+ multiple myeloma cells.a Peripheral blood mononuclear cells or bone marrow mononuclear cells from multiple myeloma (MM) patients were obtained from MM patients according to the guidelines of the Ethics Committee of the Discovery Life Sciences (Huntsville, AL, USA) and in compliance with Declaration of Helsinki protocols. Cells were thawed and measured for viability/density, and 200,000 live cells were seeded to assay plates. MM patient cells were treated with daratumumab, isatuximab (ISA) analog, or TAK-079 analog at specified concentrations in the presence of 10% human complement. After 3 days, MM cell numbers were measured using Precision Count BeadsTM (BioLegend) and by gating on live CD19-CD20–CD138+CD38+ (HuMab; does not compete with tested CD38 monoclonal antibodies). The percent cytotoxicity was determined relative to the corresponding IgG1 control. Complement was present in each experiment. Data are shown as representative experiment at 10 mg/mL treatment. aPeripheral blood mononuclear cells or bone marrow–derived macrophages from MM patients (n=5 donors).