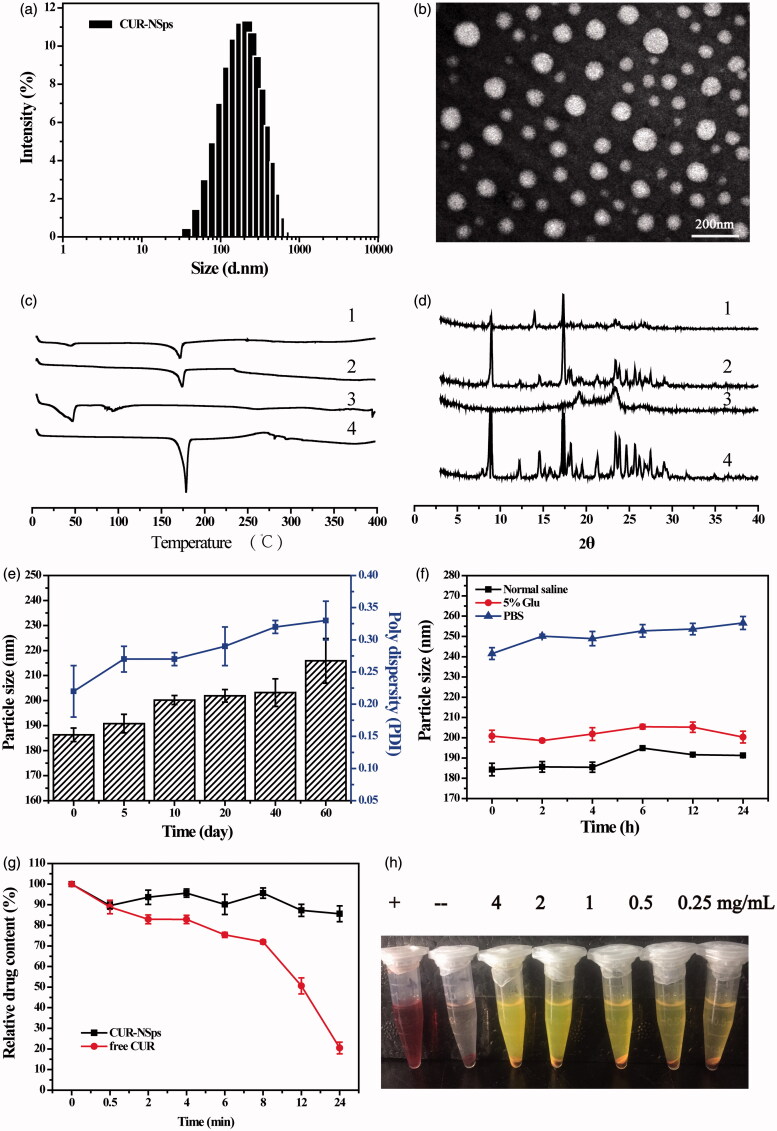
Figure 2.
Physical characterization and the stability of CURs-NSps. (a) The particle size of CUR-NSps measured by DLS. (b) TEM micrograph of CUR-NSps. The scale bar is 200 nm. (c) DSC thermograms for 1: CUR-NSps; 2: physical mixture of CUR and excipients; 3: blank excipients; 4: CUR bulk powder. (d) The XRD patterns of 1: CUR-NSps; 2: physical mixture of CUR and excipients; 3: blank excipients; 4: CUR bulk powder. (e) Particle size change of CUR-NSps during the storage at 4 °C for 60 days. (f) Particle size changes of CUR-NSps in normal saline, 5% Glu and PBS at 37 °C. (g) Relative drug content of CUR-NSps and free CUR incubated with plasma at 37 °C. (h) The hemolysis assay of CUR-NSps at different concentrations. From left to right: positive control, negative control, 4 mg/mL, 2 mg/mL, 1 mg/mL, 0.5 mg/mL, 0.25 mg/mL of CUR-NSps.