FIGURE 6.
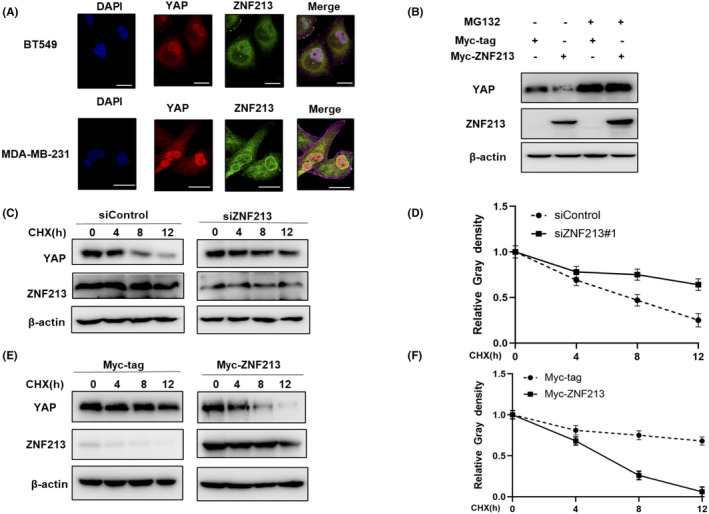
ZNF213 modulates YAP protein stability. A, Intracellular localization analysis of ZNF213 and YAP through the immunofluorescence assay. BT549 cells and MDA‐MB‐231 cells grown in normal medium before fixation. Intracellular localization of YAP (red) and ZNF213 (green) were shown in the pictures. Nuclei (blue) were stained with 40,6‐diamidino‐2‐phenylindole (DAPI). Scale bar, 20 μm. B, If the cells were treated with the proteasome inhibitor MG132, the degradation effect of ZNF213 on YAP did not increase the levels of YAP protein any more. HEK293 cells were transfected with 0.5 mg Myc‐tag or Myc ‐ZNF213 plasmids. About 24h later, cells were treated with 10 mM MG132 or DMSO for 6 h. The cell lysates were used for the analysis of western blotting. The results represent three independent experiments. C‐D, ZNF213 depletion increased the half‐life of YAP in BT549 cells. BT549 cells were transfected with 50 nM siControl or siZNF213. About 24h later, cells were treated with 100 mM cycloheximide or vehicle for the indicated times. The cell lysates were used for the analysis of western blotting. The results represent three independent experiments. Image J software was used to measure the protein density. E‐F, ZNF213 decreased YAP half‐life in HEK293 cells. HEK293 cells were transfected with 0.5 mg Myc‐tag or Myc‐ZNF213 plasmids. About 24h later, cells were treated with 100 mM cycloheximide or vehicle for the indicated times. The cell lysates were used for the analysis of western blotting. The results represent three independent experiments. Image J software was used to measure the protein density
