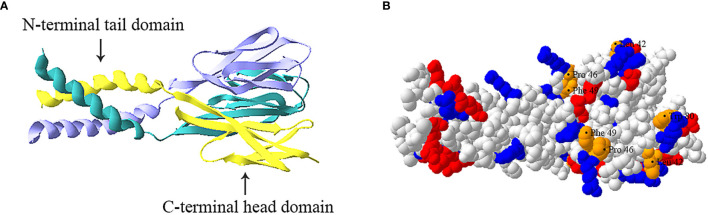
Figure 1.
Trimeric structure of human resistin. (A) Ribbon model of resistin trimer showing a C-terminal globular domain containing antiparallel β-sheets and an N-terminal α-helical domain. The three chains are indicated in cyan, yellow and lavender respectively. (B) Space-filled model of human resistin trimer. The basic (positively charged) amino acids, acidic (negatively charged) amino acids, and amino acid residues (Leu42, Pro46, Phe49, and Trp80) constituting the hydrophobic surface of the “head” region are shown in blue, red, and orange respectively. The structure was predicted based on the structure of mouse resistin structure (Protein Data Bank ID code 1RFX) and constructed by using the SWISS-MODEL program (https://swissmodel.expasy.org).