FIG 2.
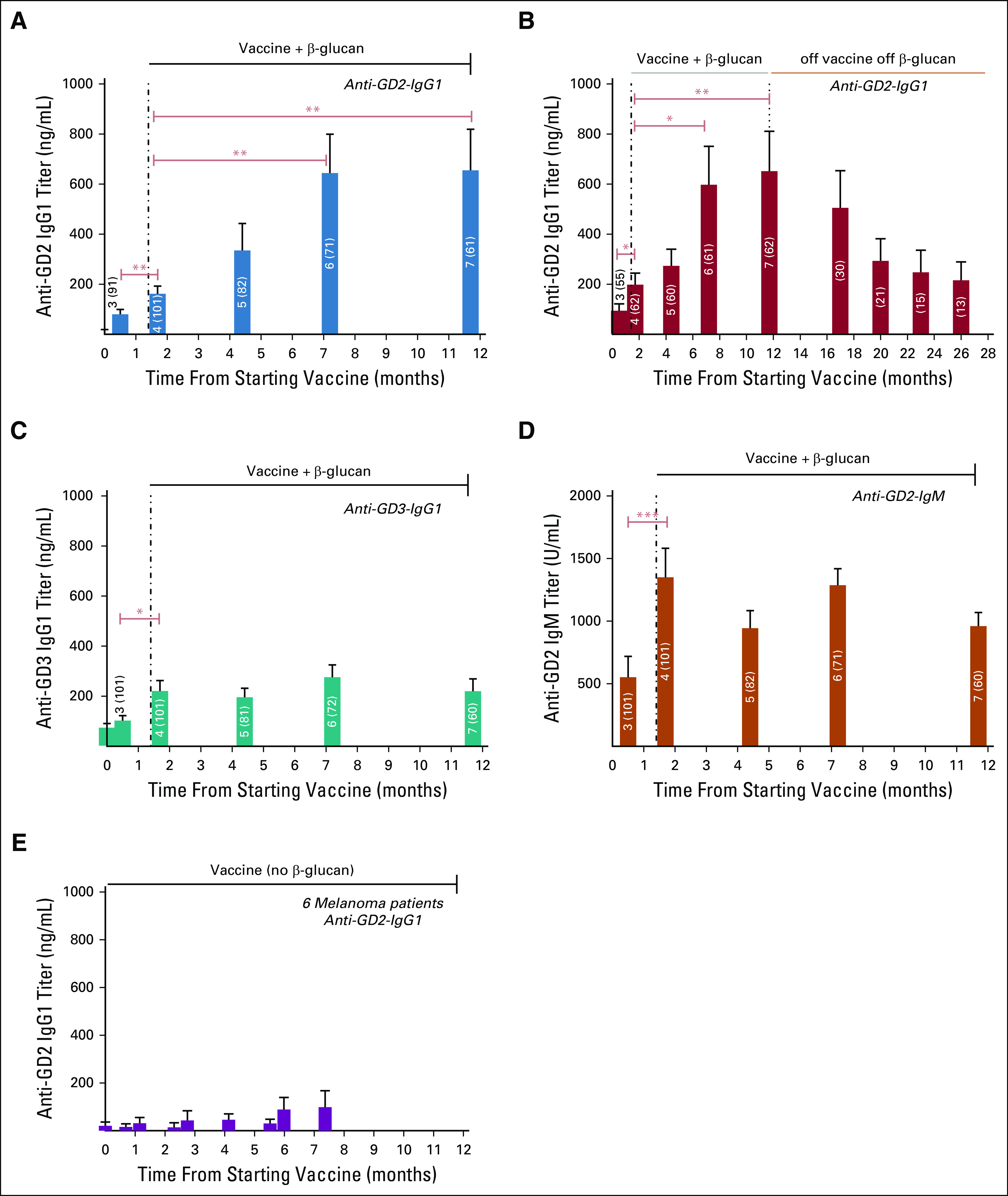
Antibody titer response during vaccine treatment among 101 patients with high-risk neuroblastoma with prior PD. One patient was excluded from this analysis because of progression before receiving β-glucan. β-glucan was initiated from week 6 to the end of vaccine (dose #7 or PD); number of each bar denotes dose #, and number in brackets denotes sample size; antibody titers were quantified by ELISA and expressed as mean + SEM; *P ≤ .05, **P ≤ .01, and ***P ≤ .001 by Wilcoxon signed-rank tests. During vaccine therapy: Anti-GD2-IgG1 titer (A), anti-GD3-IgG1 (C), and anti-GD2-IgM (D); persistence of anti-GD2-IgG1 titer among 62 patients who completed seven doses of vaccine therapy and off β-glucan (B). Anti-GD2-titer of six patients with melanoma receiving a comparable vaccine treatment that did not include oral β-glucan (E).35 PD, disease progression.
