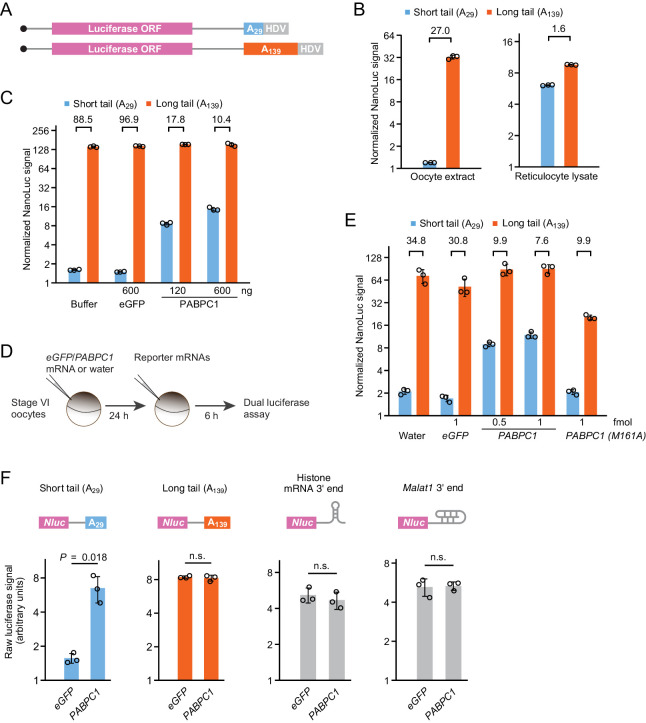
Figure 1. PABPC overexpression uncouples poly(A)-tail length and TE in frog oocytes.
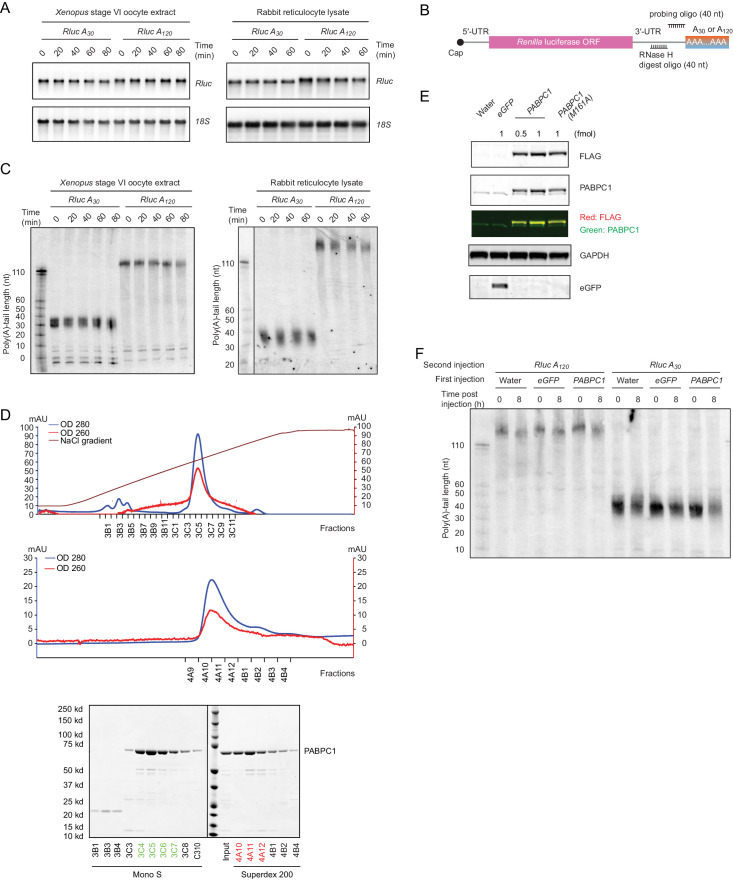
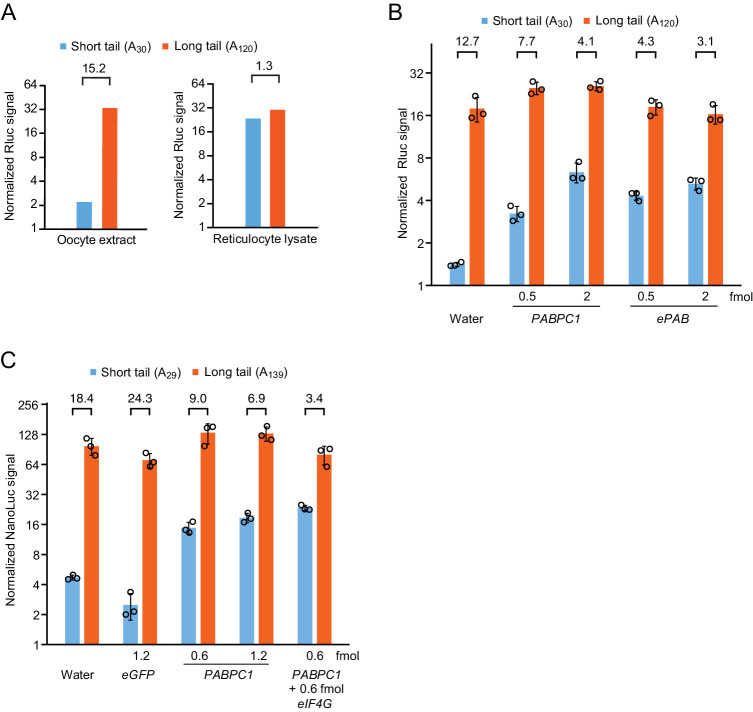
(A) Schematic of capped T7 transcripts with two different tail lengths, which were used as reporter mRNAs. Additional sequences beyond the HDV sequence are not depicted. (B) The effect of tail length on relative yields of in vitro translation of short- and long-tailed Nluc reporter mRNAs, in either frog oocyte extract (left) or rabbit reticulocyte lysate (right). The number above each bracket indicates the fold difference of the mean normalized luciferase signal (error bars, standard deviation from three technical replicates). (C) The effect of purified PABPC1 on relative yields of in vitro translation of short- and long-tailed Nluc reporter mRNAs in frog oocyte extract. Purified eGFP and PABPC1 were each added as indicated. Otherwise, this panel is as in (B). (D) Experimental scheme for serial-injection of mRNAs into frog oocytes. (E) The effect of overexpressing PAPBC1 and a PABPC1 M161A mutant on relative translation of Nluc reporter mRNAs in frog oocytes. Differential PABPC1 expression was achieved by injecting the indicated amount of mRNA in the first injection (error bars, standard deviation from three biological replicates). Otherwise, this panel is as in (B). (F) The effect of PAPBC1 overexpression on translation of reporter mRNAs with different 3′-end structures in frog oocytes. Shown are raw luciferase yields from Nluc reporters that have either a short poly(A) tail, a long poly(A) tail, a histone mRNA 3′-end stem-loop, or a Malat1 triple-helix 3′-end in oocytes overexpressing either eGFP or PABPC1 (error bars, standard deviations from three biological replicates). p values are from one-sided t-tests (n.s., not significant). For overexpression, 2.4 fmol mRNA was injected per oocyte.