Abstract
Stroke is the second most common cause of death globally and the leading cause of death in China. The pathogenesis of cerebral ischemia injury is complex, and oxidative stress plays an important role in the fundamental pathologic progression of cerebral damage in ischemic stroke. Previous studies have preliminarily confirmed that oxidative stress should be a potential therapeutic target and antioxidant as a treatment strategy for ischemic stroke. Emerging experimental studies have demonstrated that polyphenols exert the antioxidant potential to play the neuroprotection role after ischemic stroke. This comprehensive review summarizes antioxidant effects of some polyphenols, which have the most inhibition effects on reactive oxygen species generation and oxidative stress after ischemic stroke.
1. Introduction
Stroke is the second most common cause of death globally and the leading cause of death in China [1, 2]. Approximately 71% of all stroke cases are ischemic stroke, and the proportion in the developed countries is estimated to be higher, reaching up to 85% [3]. Ischemic stroke is a group of acute cerebral vascular diseases caused by various reasons leading to the interruption of cerebral arterial blood flow and the corresponding brain tissue ischemia necrosis, resulting in the loss of neural function [4]. Ischemic stroke had a high incidence and mortality rate [5, 6], which seriously affects patient life quality and brings heavy mental and economic burden to the family of patients. Both diagnosis and treatment of stroke present enormous challenges. Stroke relevant biomarkers provide an important reference for the diagnosis and prognosis of stroke. An emerging study has found that irisin was an independent prognostic marker of ischemic stroke patients, whose decreased concentration is associated with poor outcome of patients [7]. Oxidative stress and inflammation related biomarkers from saliva of stroke patients also have drawn much attention of researchers [8].
Intravenous tissue plasminogen activator (tPA) thrombolytic therapy remains the only FDA-approved emergency drug treatment within 4.5 hours after acute ischemic stroke [9]. However, the increased risk of intracerebral haemorrhage and a short treatment time window limit tPA clinical application wildly [10, 11]. At present, defibrillating therapy, antiplatelet therapy, anticoagulant therapy, and neuroprotective therapy are all reported as the potential treatment of ischemic stroke. Still, all those therapies need more clinic evidence to confirm the efficacy. Therefore, it is of great significance to accelerate research and drug development on ischemic stroke to reduce the mortality rate and improve the life quality.
The pathogenesis of cerebral ischemia injury is complex; excitatory neurotoxicity, calcium overload, oxidative/nitrosative stress, and mitochondrial dysfunction are involved the main mechanisms of cerebral ischemia injury [12]. Cerebral ischemia induces cascade reactions with the overproduction of reactive oxygen species (ROS). Inherent antioxidant potential cannot neutralize ROS and keep the endogenous redox balance, which will cause oxidative stress. Oxidative stress plays an essential role in the fundamental pathologic progression of cerebral damage in ischemic stroke [13]. When oxidative stress occurs, ROS oxidizes lipids, proteins, and nucleic acids to damage cerebral tissue structure and cells [14, 15]. Oxidative stress can also cause neuronal apoptosis, inflammation, and blood brain barrier impairment, all of which will aggravate cerebral injury after ischemic stroke [16, 17]. Previous studies have preliminarily confirmed that oxidative stress should be a potential therapeutic target and antioxidant as a treatment strategy for ischemic stroke, even though the results of clinical trials are underwhelming [18, 19]. In recent years, polyphenols are the interesting natural products (from dietary vegetables, fruits, herb medicines, and so on) because of their beneficial effects on human health and diseases. Emerging experimental studies have demonstrated that polyphenols exert the antioxidant potential to play the neuroprotection role after ischemic stroke [20]. This comprehensive review summarizes antioxidant effects of some polyphenols, such as flavonoids, phenolic acids, curcuminoids, stilbenoids, and lignans, which have the most inhibition effects on ROS generation and oxidative stress after ischemic stroke.
2. Sources and Classes of Polyphenols
Just as its name implies, polyphenols are compounds characterized by more than one phenolic hydroxyl group. However, more views do not exclude compounds with only one phenolic hydroxyl group [21]. As secondary plant metabolites, polyphenols are mainly distributed in the bark, root, leaf, shell, and fruit of a plant. Thus, polyphenols are ubiquitous in daily necessities taking plants as raw materials, including vegetables, fruits, herb medicines, tea, red wine, part of food additives, and cosmetics.
Among various natural products, flavonoids, phenolic acids, curcuminoids, stilbenoids, and lignans usually belong to polyphenols. However, not all natural products from these classifications meet the criteria for containing one or more phenolic hydroxyl groups. For instance, most of dibenzocyclooctene lignans from Schisandra chinensis, such as schisandrin, schisandrin B, schisandrin C, schisantherin A, schisandrol B, and gomisin G, have no hydroxyl group at benzene rings [22]. Figure 1 shows the chemical structures and plant origin of some common polyphenols and their classifications. It is worth mentioning that there are several subclasses as well. Flavonols, flavanones, flavones, flavan-3-ols, isoflavones, anthocyanins, dihydrochalcones, and proanthocyanidins all belong to the classification of flavonoids [23, 24]. Nevertheless, this article focuses on the antioxidant activity of polyphenols in ischemic stroke. Thus, Figure 1 does not distinguish compounds between these subclasses. In addition to ubiquitous in daily life, polyphenols are also known for their antioxidant property, which is also a core topic of discussion by nutritionists and medical workers [25].
Figure 1.
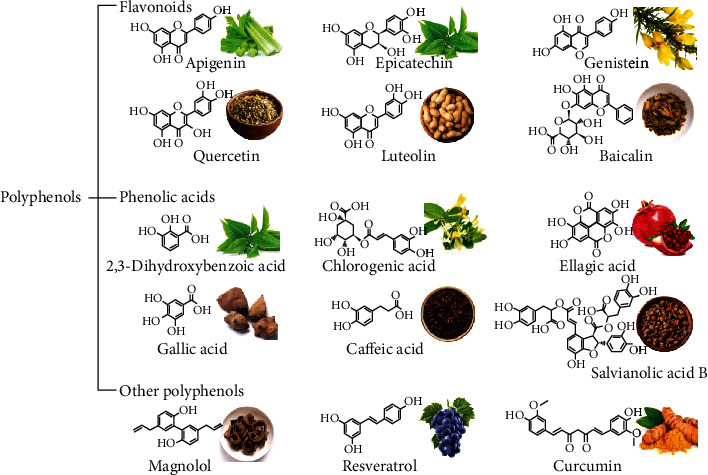
The chemical structures and plant origin of some common polyphenols and their classifications.
3. Flavonoid
Flavonoids are among the most common natural products and widely present in various plants, including vegetables, fruits, and herbs. As dietary ingredients, cohort and case-control epidemiological studies confirmed that flavonoids could reduce the risk of cardiovascular disease and other chronic diseases [26]. More importantly, flavonoids are also primary active ingredients of many herbal medicines such as Scutellaria baicalensis and Pueraria Lobata, which showed significant pharmacological activities. Based on modern medicine studies, antioxidant activity is a relatively common effect of these flavonoids [27]. Thus, in traditional Chinese medicine prescription, antioxidant activity of flavonoids plays a more or less important role in various diseases. For instance, Angong Niuhuang Pill is widely used in the treatment of ischemic stroke clinically, and baicalin is proved to be a vital ingredient extracted from Scutellaria baicalensis, one of the 12 kinds of traditional Chinese medicines that make up Angong Niuhuang Pill [28]. This instance shows us the pharmaceutical value of flavonoids as antioxidant supplementation in ischemic stroke. Thus, in this section, antioxidant action and mechanism of each flavonoid monomer in treating ischemic stroke would be enumerated and discussed.
3.1. Baicalein and Baicalin
Baicalein is a flavone with three hydroxy groups at positions C-5, C-6, and C-7. Baicalin is the 7-O-glucuronide of baicalein. The same as baicalin mentioned in a previous paragraph, baicalein is also an essential active ingredient from Scutellaria baicalensis [29]. Directly, baicalein could protect middle cerebral artery occlusion (MCAO) rats from ischemia/reperfusion (I/R) injury to some degree [30, 31]. Neurological severity, infarct volume, brain water content, and Evans blue leakage level proved the therapeutic effects of baicalein on ischemic stroke as well [30, 31]. Additionally, many common indicators related to oxidative stress suggested that antioxidant action of baicalein played a significant role. Reactive oxygen species (ROS), malondialdehyde (MDA), and 8-hydroxy-2′-deoxyguanosine (8-OhdG) were reduced, while the levels of NADPH, quinone oxidoreductase-1 (NQO1), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT) were significantly increased after baicalein treatment [30]. The activation of adenosine 5′-monophosphate- (AMP-) activated protein kinase (AMPK) and nuclear factor E2-related factor 2 (Nrf2) signaling pathways is the crucial mechanism for this antioxidant effect [30]. And upregulation of mitochondrial membrane potential (MMP) might be another mechanism that baicalein prevents neuronal cells from oxidative stress injury [31]. Mouse hippocampal neuronal cell line HT22 and toxic material iodoacetic acid were applied to establish oxidative injury cell model, whose cell survival rate could be improved by baicalein [32].
Baicalin exhibited a similar antioxidant effect as baicalein to ischemic stroke, which could also be speculated by their identical chemical structure skeleton. In baicalin research, mitochondrial succinate dehydrogenase (SDH) was a novel enzyme, which could stimulate excessive ROS production and abnormal glutamine synthetase degradation in astrocytes of ischemic stroke rats [33]. Baicalin was able to prevent astrocytes from oxidative stress via inhibiting SDH [33]. Additionally, baicalin could downregulate the levels of superoxide and peroxynitrite [34].
3.2. EC, ECG, and EGCG
(-)-Epicatechin (EC), (-)-epicatechin gallate (ECG), and (-)-epigallocatechin gallate (EGCG) are three representative polyphenols from green tea. As shown in Figure 1, the chemical structure of EC is the most simple, ECG is an ester derived from a (-)-epicatechin and a gallic acid. Very similar to ECG, EGCG is another ester with one more hydroxyl group. These tea polyphenols exert ubiquitous antioxidant effects as tea is a standard beverage in our daily life. Researchers also have explored their potential in treating ischemic stroke and indicated that EGCG was a promising antioxidant supplement or ancillary drug in stroke treatment. Several pieces of literature reported antioxidative and other roles of EC and ECG as well.
EGCG exhibited significant protective effects on various MCAO animal models. Neurologic severity score, infarct volume, and expression of apoptosis-related proteins collectively proved this effect [35–38]. ROS, MDA, SOD, CAT, and other oxidative stress-related indicators all revealed antioxidant activity of EGCG [35–38]. The activation of Nrf2-antioxidant responsive element (ARE) signaling pathway might be the main mechanism of antioxidant activity of EGCG. Nrf2-ARE is a classical antioxidant signaling pathway, and many natural products target it to treat nervous system diseases [22]. EGCG was reported to upregulate Nrf2 expression, thereby mitigating oxidative stress damage and promoting angiogenesis [35, 36]. The function and mechanism of EC in treating ischemic stroke were both analogous to EGCG, and the detailed information can be found in Table 1. ECG could protect human brain microvascular endothelial cells (HBMECs) from oxygen-glucose deprivation/reoxygenation- (OGD/R-) induced injury, while inhibition of autophagy and promotion of angiogenesis contributed to this protective effect [39]. Antioxidation also supported this protective effect, and the reduction of ROS provided direct evidence [39].
Table 1.
Antioxidant activity of flavonoids in ischemic stroke related studies.
| Flavonoids | Cell/animal model | Dosages and methods of administration in animal models | Antioxidation-related indexes | Ref |
|---|---|---|---|---|
| Baicalein | OGD cell model (SH-SY5Y) MCAO rat model (Wistar) |
In vitro: 0.1, 0.25, 0.5, 1, 2, 4, and 8 μM for 12 h In vivo: 2.5, 5, and 10 mg/kg, 7 times for 3 days before surgery, intragastric administration, tail vein injection. |
Up: NQO1, Nrf2, GSH-Px, SOD, GSH, and CAT Down: ROS, AMPK, MDA, and 8-OhdG |
[30] |
| OGD cell model (SH-SY5Y) MCAO rat model (SD) |
In vitro: 1, 5, 10, 15, and 20 μM for 12 h In vivo: 30 mg/kg; daily for 7 days, intragastric administration. |
Up: MMP | [31] | |
| Iodoacetic acid-induced oxidative injury cell model (HT22) MCAO New Zealand white rabbit model |
In vitro: 1, 2, 5, and 10 μM for 20 h In vivo: 100 mg/kg, subcutaneous injection |
Up: cell survival | [32] | |
|
| ||||
| Baicalin | Primary rat astrocytes and cortical neurons MCAO rat model (SD) |
In vitro: 0.1 to 100 μM In vivo: 100 mg/kg, intraperitoneal injection |
Down: SDH and ROS | [33] |
| OGD cell model (SH-SY5Y) MCAO rat model (SD) |
In vitro: 0.1, 1, and 10 μM for 2 h In vivo: 10, 25, and 50 mg/kg, intravenous administration |
Down: superoxide and peroxynitrite | [34] | |
| Transient global ischemia Mongolian gerbil model | 50, 100, and 200 mg/kg; daily for 7 days, intraperitoneal injection | Up: SOD, GSH, and GSH-Px Down: MDA |
[77] | |
|
| ||||
| EC | H2O2 and tert-butyl hydroperoxide-simulatedmice embryonic cortical neuronal cells MCAO mouse model (C57BL/6) |
In vitro: 0.1, 1, 10, and 100 μM for 6 h In vivo: 2.5, 5, 15, and 30 mg/kg, intragastric administration |
Up: HO-1 and nuclear Nrf2 Down: cytoplasmic Nrf2 |
[78] |
| OGD cell model (primary mice cortical neurons) MCAO mouse model (C57BL/6) |
In vitro: 50 and 100 μM In vivo: 5, 10, and 15 mg/kg, intragastric administration |
Up: HO-1, FTL, and BVR | [79] | |
|
| ||||
| ECG | OGD cell model (HBMECs) | 0.5, 1, 2, and 4 μM | Up: SOD Down: ROS and MDA |
[39] |
|
| ||||
| EGCG | MCAO rat model (SD) | 20 mg/kg, intraperitoneal injection. | Up: GSH-Px and SOD Down: NO and MDA |
[37] |
| MCAO mouse model (C57BL/6) | 50 mg/kg, intraperitoneal injection. | Up: Nrf2 and SOD1 Down: GRP78, CHOP, and Caspase 12 |
[35] | |
| Dimethylarginine-induced HBMECs injury | 20, 40, 60, 80, and 100 μM for 24 h | Down: ROS and MDA | [80] | |
| MCAO rat model (SD) | 40 mg/kg; daily for 3 days, intraperitoneal injection. | Up: GSH, Nrf2, HO-1, GCLC, and GCLM Down: ROS |
[36] | |
| MCAO rat model (SD) | 10 mg/kg; one time for 1 h before surgery and daily for Day 4 to Day 7 after surgery, intragastric administration | Up: GSH and SOD Down: NO and MDA |
[38] | |
| Glutamate-induced oxidative injury cell model (HT-22) | 1, 10, 50, and 100 100 μM for 10 h | Up: HO-1 Down: ROS |
[81] | |
|
| ||||
| Quercetin | OGD cell model (hippocampal slices and neuron/glia cultures) MCAO rat model (SD) |
In vitro: 10 μM In vivo: 20 mg/kg; daily for 21 days before surgery, intragastric administration |
Up: HO-1 Down: MDA |
[41] |
| MCAO rat model (SD) | 10 mg/kg 30 mins before surgery, intraperitoneal injection | Oxidative stress-related proteins | [82] | |
| MCAO gerbil model | 20 mg/kg 30; daily for 21 days before surgery, intragastric administration. | Up: SOD1, SOD2, CAT, and GSH-Px | [83] | |
| MCAO rat model (SD) | 30 mg/kg 30; daily for 14 days, intraperitoneal injection. | Up: GSH, GSH-Px, and GRx Down: lipid peroxidation level |
[84] | |
| MCAO rat model (SD) | 30 mg/kg 30 mins before surgery, 0, 24, 48, and 72 h after surgery, intraperitoneal injection | Up: GSH, GR, GSH-Px, GST, SOD, and CAT | [85] | |
|
| ||||
| Astragaloside IV | OGD cell model (SH-SY5Y) MCAO rat model (SD) |
In vitro: 10, 30, and 60 μg/mL In vivo: 20 mg/kg, intraperitoneal injection |
Up: SOD Down: ROS and MDA |
[86] |
| OGD cell model (neurons) | 6.25, 12.5, and 25 μmol/L 3 h before OGD and 24 h after OGD | Up: mitochondrial potential, ATP Down: ROS |
[46] | |
| TIA mouse model (C57BL/6L) | 50 mg/kg two times every day for 12 weeks, intragastric administration | Up: T-AOC, SOD, GSH Down: NOX2/4, ROS, and MDA |
[45] | |
| LPS stimulated bEnd.3 cells and C57BL/6 mice | In vitro: 25, 50 and 100 μM In vivo: 12.5, 25, and 50 mg/kg daily for 7 days, intraperitoneal injection |
Up: Nrf2, HO-1, and NQO1 Down: ROS |
[44] | |
| OGD cell model (murine cortical neurons) | 1, 10, 25, and 50 μM | Up: HO-1, NQO1, and SRXN1 Down: ROS |
[87] | |
| MCAO mouse model (C57/B6) | 20 and 40 mg/kg 0, 24, and 48 h after surgery, intraperitoneal injection | Up: GSH-Px and SOD Down: MDA |
[43] | |
|
| ||||
| Genistein | MCAO rat model (SD) | 10 mg/kg daily for 7 days before surgery, intraperitoneal injection | Up: Nrf2 and NQO1 Down: ROS |
[47] |
| MCAO rat model (SD) | 10 mg/kg 5 mins after surgery, intraperitoneal injection | Up: SOD and Nrf1 Down: MDA |
[88] | |
| H2O2-stimulated primary neurons | 0.01, 0.1, and 1 mM | Down: ROS | [48] | |
| MCAO mouse model (C57/BL6J) | 2.5, 5, and 10 mg/kg daily for 14 days before surgery, intragastric administration | Up: SOD and GSH-Px Down: MDA and ROS |
[49] | |
|
| ||||
| Rutin | Retinoic acid-induced IMR32 cell differentiation | 0.1 μM, 10 μM, and 100 μM | Down: ROS | [51] |
|
| ||||
| Hesperidin | MCAO mouse model (C57BL/6J) | 100 mg/kg daily for 10 days, intraperitoneal injection | Up: GSH, CAT, SOD, and GSH-Px Down: TBARS |
[52] |
| MCAO rat model (Wistar) | 50 mg/kg daily for 15 days before surgery, intragastric administration. | Up: GSH, CAT, SOD, GR, and GSH-Px Down: TBARS |
[53] | |
|
| ||||
| Neohesperidin | MCAO rat model (SD) | 40 mg/kg daily for 21 days before surgery, intraperitoneal injection. | Up: T-AOC, GSH, SOD, CAT, GSH-Px, GR, POD, Nrf2, and HO-1 Down: MDA, ROS, and MPO |
[54] |
|
| ||||
| Apigenin | OGD cell model (PC12) | 1, 10, and 20 μM for 6 h | Up: Nrf2, SOD, GSH-Px, CAT, MMP Down: ROS |
[59] |
| CoCl2-induced PC12 MCAO rat model |
In vitro: 1-200 μg/mL for 1 h In vivo: 25 mg/kg daily for 7 days, intraperitoneal injection |
Up: MMP Down: ROS |
[60] | |
|
| ||||
| Isoquercetin | OGD cell model (primary hippocampal neurons) MCAO rat model (SD) |
In vitro: 20, 40, and 80 μg/mL In vivo: 5, 10, and 20 mg/kg daily for 3 days, intragastric administration |
Up: SOD Down: MDA |
[62] |
| OGD cell model (primary hippocampal neurons) MCAO rat model (SD) |
In vitro: 25, 50, and 100 μg/mL In vivo: 50 mg/kg daily for 7 days, intravenous administration |
Up: SOD and Nrf2 Down: ROS |
[63] | |
| MCAO rat model (SD) | 5, 10, and 20 mg/kg daily for 3 days, intragastric administration | Up: SOD and CAT Down: ROS and MDA |
[89] | |
|
| ||||
| Isorhamnetin | MCAO mouse model (ICR) | 0.5 and 5 mg/kg, 0 and 24 hours after reperfusion, intraperitoneal injection | Up: Nrf2 and HO-1 Down: ROS and MDA |
[90] |
| Methylglyoxal plus OGD cell model (HBMECs) | 10 to 100 μM | Up: GSH Down: ROS |
[64] | |
|
| ||||
| Phloretin | MCAO rat model (SD) | 20, 40, and 80 mg/kg daily for 14 days before surgery, intraperitoneal injection | Up: Nrf2, SOD, GSH, and GSH-Px Down: MDA |
[65] |
|
| ||||
| Biochanin A | MCAO rat model (SD) | 10, 20, and 40 mg/kg daily for 14 days before surgery, intraperitoneal injection | Up: SOD, GSH-Px, Nrf2, and HO-1 Down: MDA |
[66] |
|
| ||||
| Tangeretin | OGD cell model (HBMECs) | 2.5, 5, 10, and 20 μM | Up: SOD Down: ROS, MDA, iNOS, and NO |
[55] |
|
| ||||
| Morin | MCAO rat model (Wistar) | 30 mg/kg, intraperitoneal injection | Down: ROS and MDA | [67] |
|
| ||||
| Breviscapine | MCAO rat model (SD) | 20, 50, and 100 mg/kg daily for 7 days before surgery, intraperitoneal injection | Up: SOD, GSH, and CAT Down: MDA |
[68] |
|
| ||||
| Hispidulin | MCAO rat model (Wistar) | 50 mg/kg daily for 7 days, intraperitoneal injection | Up: Nrf2, SOD, GSH-Px, and CAT Down: MDA, ROS |
[69] |
|
| ||||
| Myricetin | MCAO rat model (SD) | 1, 5, and 25 mg/kg daily for 7 days before surgery, intragastric administration | Up: SOD and GSH Down: MDA |
[91] |
| OGD cell model (SH-SY5Y) MCAO rat model (SD) |
In vitro: 0.1, 0.33, 1, 3.3 and 10 nM In vivo: 5, 10, and 20 mg/kg 2 h before surgery and 24 h, 48 h after surgery, intragastric administration |
Up: Nrf2, HO-1, SOD, CAT, mitochondrial ATP, and MMP Down: ROS, MDA, mitochondrial ROS, and mitochondrial MDA |
[70] | |
|
| ||||
| Xanthoangelol | MCAO rat model (SD) | 50 and 100 mg/kg daily for 3 days, intraperitoneal injection | Up: Nrf2, CAT, SOD and GSH-Px Down: MDA |
[71] |
|
| ||||
| Kaempferol | MCAO rat model (SD) | 1.75, 3.49, and 6.99 mM daily for 7 days before surgery, intragastric administration | Up: Nrf2, SOD and GSH Down: MDA |
[72] |
|
| ||||
| Naringenin | OGD cell model (cortical neurons) MCAO rat model (SD) |
In vitro: 20, 40 and 80 μM In vivo: 80 μM, intraperitoneal injection |
Up: SOD and Nrf2 Down: MDA and ROS |
[57] |
|
| ||||
| Chrysin | MCAO mouse model (C57/BL6) | 30 mg/kg, intraperitoneal injection | Up: SOD Down: MDA |
[92] |
| MCAO rat model (Wistar) | 10, 30, and 100 mg/kg daily for 21 days before surgery, intragastric administration | Up: GSH-Px Down: MDA, NO |
[93] | |
|
| ||||
| Icariin | Angiotensin II- (Ang II-) induced hypertension rat model | 10 mg/kg daily for 28 days, intragastric administration | Down: ROS | [73] |
|
| ||||
| Icariside II | OGD cell model (PC12) | 12.5, 25, and 50 μM | Up: MMP, Nrf2, NQO1, and HO-1 Down: ROS and Keap1 |
[74] |
|
| ||||
| Nobiletin | MCAO rat model (SD) | 10 and 25 mg/kg daily for 3 days before surgery, intraperitoneal injection | Up: Nrf2, HO-1, GSH, and SOD1 Down: MDA |
[58] |
|
| ||||
| Xanthohumol | OGD cell model (primary neurons) MCAO rat model (SD) |
0.5 μg/mL 0.4 mg/kg 10 mins before surgery, intraperitoneal injection |
Up: CAT, SOD, and Nrf2 Down: ROS, MDA, 4-HNE, 8-OhdG, and GSSSG/GSH |
[94] |
3.3. Quercetin
Quercetin is another well-known and widespread flavonol with antioxidant activity. More than 100 kinds of herbs, such as Flos Sophorae Immaturus, Cacumen biotae, and Alpinia officinarum Hance, are rich in quercetin. Vegetables such as onion, fruits such as apple, and drinks such as red wine were all detected to have a certain amount of quercetin [40]. Therefore, quercetin is a noteworthy antioxidant supplement in our daily life.
Quercetin protected against cerebral ischemia/reperfusion injury, and antioxidant activity plays a vital role [41]. Besides, quercetin suppressed lipopolysaccharide- (LPS-) induced adhesion molecule expression to treat atherosclerosis, which is a crucial induction factor of ischemic stroke. Researchers found that quercetin was able to activate Nrf2, thereby upregulating heme oxygenase 1 (HO-1) expression. Intriguingly, though adhesion molecule was under the regulation of HO-1, this phenomenon was antioxidant-independent. Nevertheless, Nrf2 activation and HO-1 upregulation actually increased antioxidants and played a role in reducing cell damage [42].
3.4. Astragaloside IV
Astragaloside IV is a principal component of a common herb clinically used for ischemic stroke: Radix Astragali. The pharmacological effect and mechanism studies of astragaloside IV to ischemic stroke are numerous and diverse [43–46]. Neurocyte protection, blood brain barrier protection, intestinal microbiota regulation, and mitochondrial function recovery were all referred to in relevant literature [43–46]. Meanwhile, antioxidant activity of astragaloside IV was correlation to all these effects.
3.5. Genistein
Genistein is a plant estrogen wildly distributed in many legumes. As a potential compound in treating ischemic stroke, genistein plays a role in neuroprotection. Researchers illuminated that genistein was able to activate Nrf2 to upregulate several antioxidase expressions [47]. So the ROS level could be decreased by genistein, and its stimulation to nuclear factor kappa-B (NF-κB), c-Jun N-terminal kinase (JNK), and extracellular regulated protein kinases (ERK) signaling pathways could be alleviated [48, 49]. As a result, nerve injury caused by inflammation and apoptosis would be partially mitigated. Stroke-prone spontaneously hypertensive rat models also suggested the antioxidant activity of genistein. Aortic endothelial cells from these rats have been detected to have a lower level of nicotinamide adenine dinucleotide phosphate (NADPH) after genistein treatment. And the authors declared that downregulation of p22phox and angiotensin II type 1 receptor expression resulted from genistein played an antioxidative role at the transcription level [50].
3.6. Other Flavonoids
Besides the flavonoids enumerated above, many flavonoids were reported to exert antioxidant function and show definite potential as supplementations in ischemic stroke (see Table 1).
Rutin is a flavonoid glycoside formed by quercetin and a disaccharide. In the differentiation process of human neuroblastoma cells (IMR32) induced by retinoic acid, rutin could decrease ROS level [51].
Hesperidin is a flavonoid found in citrus fruits, which could observably improve the content of antioxidase, such as CAT, SOD, and GSH, in rats or mouse ischemic stroke models. Cerebral injury and abnormal behavior were mitigated as well [52, 53]. Neohesperidin has the same molecular weight and very similar chemical structure as hesperidin and is also abundant in citrus fruits. Neohesperidin activated the Akt/Nrf2/HO-1 signaling pathway to inhibit oxidative stress and protect MCAO-induced brain damage [54]. Another flavonoid from citrus fruits, tangeretin, with five methoxy groups and no oxhydryl on its flavone skeleton, prevented HBMECs from OGD-induced injury via inhibiting the JNK signaling pathway. And oxidative stress injury was attenuated as well [55]. As a matter of fact, the crosstalk between JNK and Nrf2 signaling pathways plays a role in oxidative damage of stroke [56]. Naringenin, abundant in citrus fruits, exerted antiapoptotic and antioxidant effects in both the OGD/R cell model and MCAO rat model. Nrf2 gene silence or overexpression impacted these two effects, which proved the pivotal role of Nrf2 for naringenin treating ischemic stroke [57]. Another flavonoid from citrus fruits, nobiletin, showed potential in treating ischemic stroke for its anti-inflammatory and antioxidant activities [58].
Apigenin is a trihydroxyflavone widely distributed in celery and other vegetables. As a common compound with antioxidant activity, apigenin was also adopted to treat ischemic stroke as an attempt. The antioxidant function of apigenin contributed to its neuroprotective effect, and increased MMP might be a notable phenomenon [59, 60]. An apigenin flavone glycoside, vitexin, exerts stroke-treating effect primarily through reversing autophagy dysfunction. In the process of experiment, oxidative damage indexes also suggested the antioxidation of vitexin [61].
Isoquercetin is derived from quercetin with a β-D-glucosyl residue attached at position 3. Isoquercetin activated Nrf2 to exert an antioxidant effect, while toll-like receptor 4 (TLR4), NF-κB, and mitogen-activated protein kinase (MAPK) signaling pathways participated in neuroprotection as well [62]. It is worth mentioning that Nrf2 gene transcription and protein expression were both upregulated [63]. Isorhamnetin played a role in diabetic stroke. In methylglyoxal plus OGD-induced cell model (HBMECs), isorhamnetin exhibited antioxidative, anti-inflammatory, and antiapoptotic effects [64].
Phloretin is a dihydrochalcone that belongs to flavonoid, which could protect rats from ischemia/reperfusion (I/R) injury via Nrf2 activation [65]. A phytoestrogen biochanin A shows a similar effect as phloretin [66]. Morin, a pentahydroxyflavone commonly used as a natural dyestuff, could reduce ROS, inhibit lipid peroxidation (LPO), and protect blood brain barrier integrity [67]. Breviscapine, also termed scutellarin, was widely used in the clinic to exert anticoagulation and vasodilation effects. In the rat MCAO model, breviscapine could improve cognitive competence and protect the nervous system. Antioxidant and anti-inflammatory effects of breviscapine played a pivotal role [68].
Hispidulin is analogous to baicalin in chemical structure, with a methoxy group at position 6 instead of oxhydryl. Hispidulin activated Nrf2 in I/R rats through regulation of AMPK/glycogen synthase kinase-3β (GSK3β) signaling. Nrf2 gene knockdown decreased the neuroprotective effect of hispidulin, and AMPK inhibitor downregulated expression of Nrf2 [69]. Myricetin is a flavone extracted from the leaves of Myrica rubra, which could treat ischemic stroke via improving antioxidase expression and mitochondrial function. Mitochondrial ATP and MMP increased while ROS and MDA in mitochondria decreased after myricetin treatment. And researchers discovered that activating Nrf2 was the critical mechanism [70]. Xanthoangelol is an important ingredient of propolis, which triggered Nrf2 to treat ischemic stroke [71].
Kaempferol protected rats from I/R injury, p-Akt, p-GSK3β, Nrf2, p-NF-κB, and oxidative stress, and inflammation-related proteins were detected, and the levels of those protein expressions were regulated by kaempferol [72]. Two flavonoids extracted from herb Herba epimedium, icariin and icariside II, were able to scavenge ROS, respectively. Icariin activated the Nrf2/sirtuin-3 (SIRT-3) signaling pathway, while icariside II inhibited NADPH oxidase activity [73, 74]. A quercetin and an α-L-rhamnosyl moiety formed quercitrin, which reduced ischemic stroke injury via inhibiting platelet activation in arterial thrombosis. Antioxidation of quercitrin was observed as well, and inhibition of TNF receptor-associated factor 4 (TNAF4)/p47phox/Hic5 axis was the reason [75].
Sanggenon C could protect rats from MCAO injury through inhibition of ras homolog gene family. The member A/rho-associated protein kinase (RhoA/ROCK) signaling pathway benefited the anti-inflammatory and antioxidant properties of sanggenon C. The reduced efficacy of sanggenon C after RhoA overexpression illustrated this [76]. Additionally, xanthohumol, luteoloside, pinocembrin, scutellarin, silibinin, chrysin, and other flavonoids were all reported to exhibited antioxidant activity in treating ischemic stroke [21].
4. Phenolic Acid
Phenolic acids are secondary metabolic products of plants and therefore widely exist in herbs, vegetables, and fruits. Structurally, phenolic acids have one benzene ring and one or more than one phenolic hydroxyl group. In comparison to flavonoids, study progress about phenolic acids was relatively later and slower. Nevertheless, some phenolic acids, such as salvianolic acid B (from famous herb medicine Salvia miltiorrhiza) and chlorogenic acid (from Lonicera japonica), aroused great interests of researchers [95–97]. In general, phenolic acids have shown potential in antioxidation, antitumour, and antibiosis. We have highlighted the correlation between oxidative stress injury and ischemic stroke. Thus, as we expected, some phenolic acids played an antioxidative role in treating ischemic stroke.
4.1. Ferulic Acid
Natural ferulic acid often binds to polysaccharides or proteins to form the skeleton of plant cell wall [98]. Thus, many plants, such as Ferula asafoetida, Angelica sinensis, and onion, contain abundant ferulic acid. As a proverbial free-radical scavenger, ferulic acid was widely used in the food industry (antioxidant) and cosmetic industry (antiageing) [99]. In ischemic stroke, ferulic acid could decrease the content of LPO product 4-hydroxynonenal (4-HNE) and DNA oxidative damage marker 8-OhdG [100]. The researchers declared that reducing the expression of intercellular cell adhesion molecule-1 (ICAM-1) mRNA mitigated oxidative damage. With ICAM-1 inhibition, the number of microglia/macrophages decreased and thereby showed considerable anti-inflammatory action. Ultimately, inflammation-induced oxidative stress and apoptosis were ameliorated [100]. Another literature reported peroxiredoxin-2 and thioredoxin, as antioxidant protein, have a strong neuroprotective effect. Ferulic acid significantly improved the protein expression of peroxiredoxin-2 and thioredoxin detected by proteomics in the brain of MCAO rats [101].
4.2. Gallic Acid
Gallic acid is another phenolic acid applied in a vast range of foods and cosmetics. Gallic acid is a trihydroxybenzoic acid with three hydroxy groups located at positions 3, 4, and 5. In Na2S2O4-induced hypoxia/reoxygenation SH-SY5Y cells, MMP, mitochondrial ROS, ATP level, oxygen consumption, and mitochondrial permeability transition pore viability all suggested that gallic acid exhibited a powerful effect in restoring mitochondrial dysfunction. And this effect was naturally beneficial to maintain cellular redox balance [102]. Another literature reported the function of gallic acid in treating post-stroke depression. The strong correlation between behavioral parameters and antioxidant enzyme levels such as SOD and GSH before and after gallic acid treatment proved the critical role of antioxidation. Additionally, a gallic acid derivative, methyl-3-O-methyl gallate, showed better antioxidant and antidepressant effects than gallic acid [103]. This result reminded us gallic acid might be a lead compound in the pursuit of a more efficient antioxidant to treat ischemic stroke. High concentrations of particulate matter might increase risk of ischemic stroke. To dusty particulate matter exposed stroke rats, gallic acid also exerted observably antioxidant effect [104].
4.3. Salvianolic Acid B
In China, Composite Salvia Miltiorrhiza Injection is a marketed drug approved by National Medical Products Administration for cerebrovascular accident prevention and treatment. Salvianolic acid B is one of the most important ingredients of Composite Salvia Miltiorrhiza Injection [105]. A metabolomics study suggested salvianolic acid B has antioxidant function because of content changes of oxidative stress-related biomarkers [95]. Researchers also discovered that salvianolic acid B could suppress activation of astrocytes and microglia and downregulate the ROS level in MCAO mice [96].
4.4. Other Phenolic Acids
Chlorogenic acid is a depside formed by caffeic acid and quinic acid. Literatures about antibacterial, antiviral, and antioxidant effects of chlorogenic acid are numerous [106]. Researchers also made an effort to study the function of chlorogenic acid in ischemic stroke. One literature reported that chlorogenic acid dose-dependently improved learning and memory ability and alleviated brain damage of I/R rats. Proteins in the Nrf2 signaling pathway, including Nrf2, HO-1, and NQO1, were all detected at a higher level after administering chlorogenic acid. The authors also used Nrf2 inhibitor ML385 to further prove the effect of chlorogenic acid in activating the Nrf2 signaling pathway [97].
Caffeic acid could cross blood brain barrier and has multiple biological activities including antioxidation [107]. Caffeic acid could ameliorate neurological dysfunction and decrease infarct volume after focal cerebral ischemia in rats by downregulating expression of 5-lipoxygenase, an enzyme catalyzing lipid oxidation [108, 109]. In the process of arachidonic acid producing leukotrienes, 5-lipoxygenase exerted key catalysis, which exacerbated nerve damage of cerebral ischemia rats [108].
Compared to other phenolic acids, ellagic acid has a characteristic organic heterotetracyclic structure. Antioxidant and antiproliferative effects of ellagic acid are the most concerned. As an active ingredient in cranberries, strawberries, pomegranates, and other common fruits, the role of ellagic acid in ischemic stroke is also worth investigating [110]. As expected, ellagic acid showed a beneficial effect on MCAO rats through regulating the expression of zonula occludens-1 (up), aquaporin 4, and matrix metalloproteinase 9 (down). The high level of some representative antioxidant enzymes also clarified antioxidation of ellagic acid [111].
Rosmarinic acid could also upregulate Nrf2 to exert antioxidant and neuroprotective functions. Zinc protoporphyrin IX, an HO-1 inhibitor, suppressed the antioxidant and antiapoptotic effects of rosmarinic acid. And researchers discovered that phosphoinositide 3-kinase (PI3K)/Akt was an upstream regulator of Nrf2 and PI3K inhibitor LY294002 decreased Nrf2 and HO-1 expression. To sum up, rosmarinic acid protected against ischemic stroke; PI3K/Akt signaling pathway activation and following up-regulation of Nrf2 and HO-1 were the involved mechanism [112].
5. Curcuminoid
Curcumin, demethoxycurcumin, tetrahydrocurcumin, hexahydrocurcumin, and bisdemethoxycurcumin belong to curcuminoid family. Curcumin was extracted from Curcuma longa at the earliest. Curcumin had extensive and strong pharmacological effects, such as anti-inflammation, antioxidation, and antitumour [113, 114]. In recent years, more and more evidence showed that curcumin had pretty high potential in treating cardiovascular and cerebrovascular diseases [115]. And kind of literature reported the effects of curcumin against ischemic stroke.
Thiyagarajan and Sharma firstly reported that the antioxidation-mediated neuroprotective effect was why curcumin protected rats from I/R injury in 2004. Concretely, dose-dependent reduction of cerebral infarct volume and cerebral edema volume proved the effect of curcumin against ischemic stroke. Peroxynitrite formation inhibition and protein tyrosine nitration reduction in the cytosolic suggested the antioxidative role of curcumin [116]. Another literature in 2010 made a similar study and mainly focused on Caspase 3, B-cell lymphoma-2 (Bcl-2), and other apoptosis-related proteins. MDA was downregulated as an oxidative stress index as well [117].
Molecular mechanism of antioxidant activity of curcumin in ischemic stroke was complicated. AMPK/uncoupling protein 2 (UCP2) signaling pathway was referred to as UCP2 which was able to limit excess ROS. Researchers found that curcumin could upregulate p-AMPK and UCP2, by which cerebrovascular and endothelial cell dysfunction could be attenuated. AMPK inhibitor, UCP2 inhibitor, and UCP2 gene knockout all suggested significance of this pathway to antioxidation of curcumin [118, 119]. The effect of curcumin on AMPK was also related to endoplasmic reticulum stress and associated thioredoxin-interacting protein/NACHT, LRR, and PYD domain-containing protein 3 (TXNIP/NLRP3) inflammasome activation. And endoplasmic reticulum stress in this paper resulted from a high ROS level [120]. Akt/Nrf2 was also involved in the antioxidant property of curcumin. Impacting on Akt phosphorylation was regarded as the critical factor of antioxidant and neuroprotective effects of curcumin [121]. Another report highlighted the role of peroxiredoxin 6 and specific protein1 (SP1) after ischemic stroke. The peroxiredoxin 6 gene silence or SP1 antagonism would severely weaken the therapeutical effect of curcumin. In normal conditions, the number of peroxiredoxin 6-positive neuronal cells and protein expression of peroxiredoxin 6 were both increased after curcumin administration [122]. Additionally, enhancement of apurinic/apyrimidinic endonuclease 1 in level and activity by curcumin also benefited its oxidation resistance and therapeutic effect [123].
Tetrahydrocurcumin is a derivant of curcumin, with double bonds be reduced to single bonds. Researchers found that tetrahydrocurcumin also plays a role in ischemic stroke, and recovering mitochondrial dysfunction of cerebral vascular cells might be a key factor [124, 125]. Other than common indexes such as neurological score, brain edema, cerebral infarction, and blood flow, the authors also found that tetrahydrocurcumin reduced permeability of blood brain barrier and recovered abnormal homocysteine metabolism via altering several related enzymes [124]. More importantly, tetrahydrocurcumin alleviated mitochondrial oxidative stress and inhibited mitochondrial dysfunction induced by oxidative stress. Levels of thioredoxin-2, SOD2, p47phox, and gp91phox all proved the effect of tetrahydrocurcumin [124].
Hexahydrocurcumin, as one of the major metabolites of curcumin, significantly reduced the neurological deficit scores and the infarct volume in cerebral I/R injury rats. Treatment with hexahydrocurcumin significantly attenuated oxidative stress and inflammation, with decreased levels of MDA and NO and increased levels of the antioxidative enzymes and superoxide dismutase (SOD) activity in I/R rats [126]. A comparative study demonstrated that pretreated with polymeric N-isopropylacrylamide (PNIPAM) nanoformulation of curcumin, demethoxycurcumin, and bisdemethoxycurcumin intranasal delivery significantly improved neurological deficits, locomotor activity, and grip strength by decreasing the level of LPO and increasing the activities of antioxidant enzymes (GSH-Px, glutathione reductase, SOD, and CAT) in MCAO rats. These results suggest that PNIPAM-loaded curcumin nanoparticles may also be a potential neuroprotective agent against various conditions where cellular damage is a consequence of oxidative stress [127].
6. Stilbenoid
Resveratrol is a classic biologically active natural product, also a well-known and widely used antioxidant. Many literatures described stilbenoid as a nonflavonoid polyphenol. And more precisely, it was classified as a stilbenoid, which took stilbene or its polymeride as a skeleton structure. Resveratrol has both cis and trans structures, and trans structures are the abundant existing form in plants.
For patients who had a stroke within one year, oral 100-200 mg resveratrol daily for one year reduced or limited rising of blood pressure, blood glucose, blood lipid, and body mass index, which suggested resveratrol positively affected the prognosis of stroke patients [128]. As an antioxidant, resveratrol was applied to treat ischemic stroke in an experimental study as well. Sinha et al. firstly indicated that resveratrol protected MCAO rats via inhibition of oxidative stress [129]. Numerous relevant cell models and animal models were then adopted and proved the antioxidative role of resveratrol during ischemic stroke. H2O2-induced oxidative stress injury in hippocampal slice was alleviated by resveratrol, with an improved level of GST [130]. Similarly, in OGD-injured PC12 cells, bilateral common carotid artery (BCCA) occlusion induced cerebral infarction rats and diabetic rats with ischemic stroke; resveratrol exhibited antioxidant activity as well [131–133]. Enriched environment is beneficial to the recovery of brain injury and neurological dysfunction after ischemic stroke. In enriched environment, the therapeutic effect of resveratrol was improved, and the antioxidation could still be observed [134]. Hermann et al. divided the administration modes of resveratrol into prophylactic delivery, acute delivery, and postacute delivery, corresponding to administrating daily for 7 days until surgery, administrating immediately after reperfusion and 24 h after reperfusion, and administrating daily for 28 days after surgery, respectively. The result suggested that the first two administration modes were conducive to exert an antioxidant effect, while in administration after surgery group, thiobarbituric acid reactive substances (TBARS) formation and HO-1 level showed no significant difference with the control group [135].
The antioxidation mechanism for resveratrol mainly includes restoring mitochondrial function, activating sirtuin-1 (SIRT-1) and Nrf2. The significance of mitochondria to maintain redox homeostasis was mentioned above. In the oxygen and nutrient-deficient environments, excessive ROS production by the mitochondria would lead to mitochondrial lipid peroxidation and mitochondrial membrane depolarization. Thus, a vicious cycle of mitochondrial damage and oxidative stress would ultimately aggravate ischemic stroke [136]. Genomic analysis was applied in the neuronal-astrocytic coculture model to detect the gene expression differences before and after resveratrol preconditioning. TCA cycle, oxidative phosphorylation, and pyruvate uptake-related genes were upregulated. ATP level, glycolysis, and mitochondrial respiration efficiency were observed to be increased by resveratrol as well. All this evidence collectively illuminated the protection of resveratrol from oxygen and nutrient-deficient induced mitochondrial dysfunction [137, 138].
SIRT-1 is a deacetylase that regulated various biological functions of substrate proteins by deacetylation. Cell metabolism and apoptosis were involved in the regulating effect of SIRT-1 [139]. Concretely and correlatively, mitochondrial biosynthesis and fatty acid oxidation regulated by SIRT-1 were concerned in this paper [140]. Resveratrol activated SIRT-1, thereby protecting endothelium of the cerebrovasculature from oxidative stress injury, and ultimately exhibited a curative effect on ischemic stroke. SIRT-1 inhibitor significantly blocked these functions of resveratrol [141]. In terms of mechanism, activated SIRT-1 could increase the expression of downstream protein peroxisome proliferator-activated receptor γ coactivator-1α (PGC1α) and target genes UCP2 and SOD2 [142]. Resveratrol could also activate Nrf2 and upregulate the expression of downstream proteins, including HO-1 and NQO1, which was verified by multiple research groups based on the OGD cell model and MCAO animal model [143–145].
7. Other Polyphenols
Lignan is a kind of phytoestrogen formed by two phenylpropanoid derivatives [22]. Magnolol belongs to the lignan family and exhibits antioxidant property during ischemic stroke. As shown in Figure 1, magnolol is a typical polyphenol and abundant in herb medicine Magnolia officinalis. Magnolol showed a certain degree of competence in scavenging free radicals. As for lipid peroxidation inhibition in brain tissue, magnolol exhibited strong ability, and the IC50 value of dPPH radical scavenging assay was lower than β-estradiol, α-tocopherol, and ascorbic acid. MDA and 4-HNE level, nitrate/nitrite, and myeloperoxidase (MPO) activity all verified this. A direct neuroprotective effect was reflected in neonatal rat hippocampal slice cultures, and magnolol could mitigate the damage induced by OGD [146].
8. Conclusion and Prospect
8.1. Molecular Mechanisms of Polyphenols as Antioxidant Supplementations
Based on the introduction and Table 1, up to now, reported works observed antioxidation of polyphenols via detection of ROS, antioxidation-related enzymes such as SOD, CAT, GSH, and GSH-Px and oxidative stress byproducts such as MDA, 4-HNE, and 8-OhdG. As for molecular mechanisms, Nrf2-ARE was a recognized signaling pathway under regulation of polyphenols, including flavonoids, phenolic acids, curcuminoids, and stilbenoids. Specifically, curcumin-activated AMPK/UCP2 signaling pathway and resveratrol-activated SIRT-1 are both beneficial to antioxidation. The regulation effects on these three pathways were all repetitively verified by specific inhibitors or gene knockout.
Figure 2 exhibits the correlation of ROS and Nrf2-ARE signaling pathway. Under condition of redox equilibrium, Nrf2 usually locates in the cytoplasm and is limited by an upstream regulator Keap1, an E3 ubiquitin ligase complex. Keap1 catalyzes ubiquitin modification of Nrf2 cooperatively with Cullin-3 (Cul3) and subsequently degraded by the 26 s proteasome. Under oxidative stress, high ROS-induced electrophile metabolites would modify nonenzymatic covalent highly reactive cysteine residues in Keap1. Ultimately, Nrf2 would be released by Keap1 and exert its biological function. As a transcription factor, Nrf2 transfers to the nucleus and forms a dipolymer with small musculoaponeurotic fibrosarcoma (sMaf), which would bind to ARE and promotes expression of a series of antioxidation-related enzymes such as HO-1 and Nqo1. And cytoplasm Nrf2 plays a role via phosphorylation, nuclear localization, and ARE binding [147–149]. In the literatures about polyphenols treating ischemic stroke, Nrf2 transcriptional, expression, and phosphorylation level were all reported to be upregulated, which illuminated the role of Nrf2 in the antioxidation of polyphenols.
Figure 2.
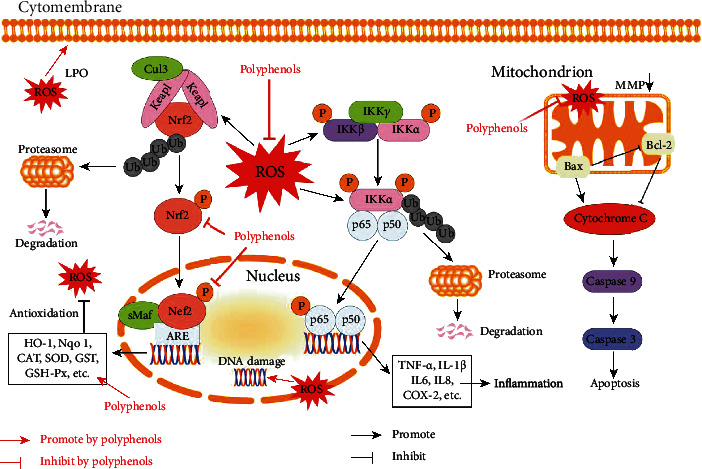
Molecular mechanisms of polyphenols as antioxidant supplementations in ischemic stroke.
The high level of ROS not only stimulates Keap-Nrf2 but also activates inflammation and apoptosis-related signaling pathways. Figure 2 also shows the crosstalk between ROS and NF-κB, a significant inflammatory regulator, which is held in a resting state through association with inhibitor of κB (IκB) proteins [150]. On the one hand, some enzymes, such as gp91 phox, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) that regulated by NF-κB, also play a role in promoting ROS. On the other hand, ROS could trigger the activation of the IκB kinase complex, leading to phosphorylation, ubiquitination, and degradation of IκB proteins, which could release anti-inflammatory factors and induce inflammation [151, 152]. Actually, in many experiments, NF-κB was observed to be activated, and various inflammatory factors such as interleukin-1β (IL-1β), interleukin-6 (IL6), interleukin-8 (IL8), and tumour necrosis factor α (TNF-α) were increased [152]. Thus, downregulation ROS effect of polyphenols was an important reason for inflammation mitigation in stroke patients.
Elevated ROS in mitochondria would initiate the apoptotic process. Concretely, as shown in Figure 2, the high level of BCL2-associated X (Bax) and the low level of Bcl-2 along with loss of MMP, leading to apoptosome formation and thereby downstream caspase cascade, ultimately induced apoptosis [153]. And the level changes of Bax, Bcl-2, MMP, and Caspase 3 were all detected in stroke cell or animal models after polyphenols administration.
8.2. Superiorities of Polyphenols as Antioxidant Supplementations
Until now, there is no efficacious drug for ischemic stroke treatment besides tPA thrombolytic therapy. Many potential compounds were abandoned after phase II or III clinical trials because of inadequate efficacy, overstrong side effects, or other reasons. Among these failed compounds, inhibition of free radical production, free radical scavenging, free radical degradation, and mitochondrial targeted antioxidation were all taken as antioxidant strategies [154]. The temporary failure of antioxidants was disappointing. Nevertheless, stroke mortality showed a downward trend, and effective prevention and rehabilitation might be a significant reason [155]. During prevention and rehabilitation of ischemic stroke, healthy diet and exercise played a nonnegligible role. As described above, as a class of natural products, polyphenols were abundant in fruits, vegetables, and drinks, which exhibited the critical advantage of polyphenols: convenient ingestion and subtle influence.
Not only exerting antioxidative function in daily life diet, some polyphenols were also applied in clinical settings for a long time. Taking baicalin for instance, its source herb Scutellaria baicalensis has been used as ischemic stroke medication in China. Other polyphenols from herb medicine might likewise play a role in ischemic stroke. Inherited clinical experience ensured the efficacy and safety of these polyphenols to a certain degree, which was another advantage of polyphenols. Nevertheless, for more extensive and more international application of herb medicine, much more effort was needed to precisely clarify the therapeutic mechanism, side effect, toxicity, drug metabolism, and many other aspects of these polyphenols as drugs.
Multiple actions and multiple targets of herb medicine have been widely recognized [156]. Though this article focused on antioxidation of polyphenols, their other pharmacologic actions such as anti-inflammation, antiapoptosis, and angiogenesis promotion were also nonnegligible. Actually, in most literatures reported, the therapeutical effects of polyphenols in ischemic stroke, inflammatory factors, and apoptosis-related proteins were observed altering as well. Though, as introduced above, excess ROS was involved in inflammation and apoptosis, some polyphenols could protect the brain from these harmful factors via oxidative stress-independent mechanisms [21]. Without a doubt, some potential therapeutic mechanisms of polyphenols in treating ischemic stroke still were waiting to be discovered. Above all, antioxidation cooperatively functioned with other relevant effects and mitigated symptoms of ischemic stroke.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81622051), the National Key Research and Development Program of China (No. 2018YFC1705005), and the Foundation of Zhejiang Chinese Medical University (No. 2020ZR20).
Data Availability
No data were used to support this review article.
Conflicts of Interest
The authors declared that there was no potential conflict of interest.
Authors' Contributions
YZ, XF, and SZ wrote and revised the manuscript. YZ drew the table and figures. All the authors read and approved the final manuscript.
References
- 1.Zhou M., Wang H., Zeng X., et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao B. H., Yan F., Hua Y., et al. Stroke prevention and control system in China: CSPPC-Stroke Program. International Journal of Stroke. 2021;16(3):265–272. doi: 10.1177/1747493020913557. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Stroke Expert Group. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. The New England Journal of Medicine. 2018;379(25):2429–2437. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kernan W. N., Ovbiagele B., Black H. R., et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 5.Yitshak Sade M., Novack V., Ifergane G., Horev A., Kloog I. Air pollution and ischemic stroke among young adults. Stroke. 2015;46(12):3348–3353. doi: 10.1161/STROKEAHA.115.010992. [DOI] [PubMed] [Google Scholar]
- 6.Mackey J., Kleindorfer D., Sucharew H., et al. Population-based study of wake-up strokes. Neurology. 2011;76(19):1662–1667. doi: 10.1212/WNL.0b013e318219fb30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu W. J., Qiu H. C., Cao J. L., Liu Q., Zeng X. W., Zhao J. Z. Decreased concentration of irisin is associated with poor functional outcome in ischemic stroke. Neurotherapeutics. 2018;15(4):1158–1167. doi: 10.1007/s13311-018-0651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maciejczyk M., Bielas M., Zalewska A., Gerreth K. Salivary biomarkers of oxidative stress and inflammation in stroke patients: from basic research to clinical practice. Oxidative Medicine and Cellular Longevity. 2021;2021:22. doi: 10.1155/2021/5545330.5545330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hacke W., Kaste M., Bluhmki E., et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England Journal of Medicine. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 10.Yepes M., Roussel B. D., Ali C., Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends in Neurosciences. 2009;32(1):48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Fan X., Yu Z., Liu J., et al. Annexin A2: a tissue plasminogen activator amplifier for thrombolytic stroke therapy. Stroke. 2010;41(10 Suppl):S54–S58. doi: 10.1161/STROKEAHA.110.596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo E. H., Dalkara T., Moskowitz M. A. Mechanisms, challenges and opportunities in stroke. Nature Reviews. Neuroscience. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 13.Chen H., Yoshioka H., Kim G. S., et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxidants & Redox Signaling. 2011;14(8):1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen C. L., Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. International Journal of Stroke. 2009;4(6):461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 15.Sun M.-S., Jin H., Sun X., et al. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxidative Medicine and Cellular Longevity. 2018;2018:17. doi: 10.1155/2018/3804979.3804979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinschnitz C., Grund H., Wingler K., et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biology. 2010;8(9) doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casas A. I., Geuss E., Kleikers P. W., et al. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(46):12315–12320. doi: 10.1073/pnas.1705034114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forrester S. J., Kikuchi D. S., Hernandes M. S., Xu Q., Griendling K. K. Reactive oxygen species in metabolic and inflammatory signaling. Circulation Research. 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjelakovic G., Nikolova D., Gluud L. L., Simonetti R. G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 20.Xu H., Wang E., Chen F., Xiao J., Wang M. Neuroprotective phytochemicals in experimental ischemic stroke: mechanisms and potential clinical applications. Oxidative Medicine and Cellular Longevity. 2021;2021:45. doi: 10.1155/2021/6687386.6687386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrella E., Gussago C., Porrini V., Benarese M., Pizzi M. From preclinical stroke models to humans: polyphenols in the prevention and treatment of stroke. Nutrients. 2020;13(1) doi: 10.3390/nu13010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y., Men L., Sun Y., Wei M., Fan X. Pharmacodynamic effects and molecular mechanisms of lignans from Schisandra chinensis Turcz. (Baill.), a current review. European Journal of Pharmacology. 2021;892, article 173796 doi: 10.1016/j.ejphar.2020.173796. [DOI] [PubMed] [Google Scholar]
- 23.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crozier A., Jaganath I. B., Clifford M. N. Dietary phenolics: chemistry, bioavailability and effects on health. Natural Product Reports. 2009;26(8):1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 25.Myburgh K. H. Polyphenol supplementation: benefits for exercise performance or oxidative stress? Sports Medicine. 2014;44(Suppl 1):S57–S70. doi: 10.1007/s40279-014-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graf B. A., Milbury P. E., Blumberg J. B. Flavonols, flavones, flavanones, and human health: epidemiological evidence. Journal of Medicinal Food. 2005;8(3):281–290. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- 27.Verma A. K., Pratap R. The biological potential of flavones. Natural Product Reports. 2010;27(11):1571–1593. doi: 10.1039/c004698c. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D., Fu M., Song C., Wang C., Lin X., Liu Y. Expressions of apoptosis-related proteins in rats with focal cerebral ischemia after Angong Niuhuang sticker point application. Neural Regeneration Research. 2012;7(30):2347–2353. doi: 10.3969/j.issn.1673-5374.2012.30.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu T., Wang L., Feng Y., Sun G., Sun X. Classical active ingredients and extracts of Chinese herbal medicines: pharmacokinetics, pharmacodynamics, and molecular mechanisms for ischemic stroke. Oxidative Medicine and Cellular Longevity. 2021;2021:27. doi: 10.1155/2021/8868941.8868941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan Y., Men W., Shan X., et al. Baicalein exerts neuroprotective effect against ischaemic/reperfusion injury via alteration of NF-kB and LOX and AMPK/Nrf2 pathway. Inflammopharmacology. 2020;28(5):1327–1341. doi: 10.1007/s10787-020-00714-6. [DOI] [PubMed] [Google Scholar]
- 31.Li W. H., Yang Y. L., Cheng X., et al. Baicalein attenuates caspase-independent cells death via inhibiting PARP-1 activation and AIF nuclear translocation in cerebral ischemia/reperfusion rats. Apoptosis. 2020;25(5-6):354–369. doi: 10.1007/s10495-020-01600-w. [DOI] [PubMed] [Google Scholar]
- 32.Lapchak P. A., Maher P., Schubert D., Zivin J. A. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150(3):585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Song X., Gong Z., Liu K., Kou J., Liu B., Liu K. Baicalin combats glutamate excitotoxicity via protecting glutamine synthetase from ROS-induced 20S proteasomal degradation. Redox biology. 2020;34, article 101559 doi: 10.1016/j.redox.2020.101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu M., Chen X., Gu Y., et al. Baicalin can scavenge peroxynitrite and ameliorate endogenous peroxynitrite-mediated neurotoxicity in cerebral ischemia-reperfusion injury. Journal of Ethnopharmacology. 2013;150(1):116–124. doi: 10.1016/j.jep.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Bai Q., Lyu Z., Yang X., Pan Z., Lou J., Dong T. Epigallocatechin-3-gallate promotes angiogenesis via up-regulation of Nfr2 signaling pathway in a mouse model of ischemic stroke. Behavioural Brain Research. 2017;321:79–86. doi: 10.1016/j.bbr.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Han J., Wang M., Jing X., Shi H., Ren M., Lou H. (-)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochemical Research. 2014;39(7):1292–1299. doi: 10.1007/s11064-014-1311-5. [DOI] [PubMed] [Google Scholar]
- 37.Nan W., Zhonghang X., Keyan C., Tongtong L., Wanshu G., Zhongxin X. Epigallocatechin-3-gallate reduces neuronal apoptosis in rats after middle cerebral artery occlusion injury via PI3K/AKT/eNOS signaling pathway. BioMed Research International. 2018;2018:9. doi: 10.1155/2018/6473580.6473580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu K. J., Hsieh M. T., Wu C. R., Wood W. G., Chen Y. F. Green tea extract ameliorates learning and memory deficits in ischemic rats via its active component polyphenol epigallocatechin-3-gallate by modulation of oxidative stress and neuroinflammation. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11. doi: 10.1155/2012/163106.163106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu B., Zeng Q., Zhang Z., et al. Epicatechin gallate protects HBMVECs from ischemia/reperfusion injury through ameliorating apoptosis and autophagy and promoting neovascularization. Oxidative Medicine and Cellular Longevity. 2019;2019:11. doi: 10.1155/2019/7824684.7824684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y. Y., Chang C. Y., Lin S. Y., et al. Quercetin protects against cerebral ischemia/reperfusion and oxygen glucose deprivation/reoxygenation neurotoxicity. The Journal of nutritional biochemistry. 2020;83, article 108436 doi: 10.1016/j.jnutbio.2020.108436. [DOI] [PubMed] [Google Scholar]
- 42.Li C., Zhang W. J., Frei B. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biology. 2016;9:104–113. doi: 10.1016/j.redox.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo Y., Qin Z., Hong Z., et al. Astragaloside IV protects against ischemic brain injury in a murine model of transient focal ischemia. Neuroscience Letters. 2004;363(3):218–223. doi: 10.1016/j.neulet.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 44.Li H., Wang P., Huang F., et al. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption _via_ activating Nrf2 antioxidant signaling pathway in mice. Toxicology and Applied Pharmacology. 2018;340:58–66. doi: 10.1016/j.taap.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Xu N., Kan P., Yao X., et al. Astragaloside IV reversed the autophagy and oxidative stress induced by the intestinal microbiota of AIS in mice. Journal of Microbiology. 2018;56(11):838–846. doi: 10.1007/s12275-018-8327-5. [DOI] [PubMed] [Google Scholar]
- 46.Xue B., Huang J., Ma B., Yang B., Chang D., Liu J. Astragaloside IV protects primary cerebral cortical neurons from oxygen and glucose deprivation/reoxygenation by activating the PKA/CREB pathway. Neuroscience. 2019;404:326–337. doi: 10.1016/j.neuroscience.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 47.Miao Z. Y., Xia X., Che L., Song Y. T. Genistein attenuates brain damage induced by transient cerebral ischemia through up-regulation of Nrf2 expression in ovariectomized rats. Neurological Research. 2018;40(8):689–695. doi: 10.1080/01616412.2018.1462879. [DOI] [PubMed] [Google Scholar]
- 48.Qian Y., Cao L., Guan T., et al. Protection by genistein on cortical neurons against oxidative stress injury via inhibition of NF-kappaB, JNK and ERK signaling pathway. Pharmaceutical Biology. 2015;53(8):1124–1132. doi: 10.3109/13880209.2014.962057. [DOI] [PubMed] [Google Scholar]
- 49.Qian Y., Guan T., Huang M., et al. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochemistry International. 2012;60(8):759–767. doi: 10.1016/j.neuint.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Xu J. W., Ikeda K., Yamori Y. Genistein inhibits expressions of NADPH oxidase p22phox and angiotensin II type 1 receptor in aortic endothelial cells from stroke-prone spontaneously hypertensive rats. Hypertension Research. 2004;27(9):675–683. doi: 10.1291/hypres.27.675. [DOI] [PubMed] [Google Scholar]
- 51.Sivanantham B., Krishnan U., Rajendiran V. Amelioration of oxidative stress in differentiated neuronal cells by rutin regulated by a concentration switch. Biomedicine & Pharmacotherapy. 2018;108:15–26. doi: 10.1016/j.biopha.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Oztanir M. N., Ciftci O., Cetin A., Aladag M. A. Hesperidin attenuates oxidative and neuronal damage caused by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model. Neurological Sciences. 2014;35(9):1393–1399. doi: 10.1007/s10072-014-1725-5. [DOI] [PubMed] [Google Scholar]
- 53.Raza S. S., Khan M. M., Ahmad A., et al. Hesperidin ameliorates functional and histological outcome and reduces neuroinflammation in experimental stroke. Brain Research. 2011;1420:93–105. doi: 10.1016/j.brainres.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 54.Wang J. J., Cui P. Neohesperidin attenuates cerebral ischemia-reperfusion injury via inhibiting the apoptotic pathway and activating the Akt/Nrf2/HO-1 pathway. Journal of Asian Natural Products Research. 2013;15(9):1023–1037. doi: 10.1080/10286020.2013.827176. [DOI] [PubMed] [Google Scholar]
- 55.Wu C., Zhao J., Chen Y., et al. Tangeretin protects human brain microvascular endothelial cells against oxygen-glucose deprivation-induced injury. Journal of Cellular Biochemistry. 2019;120(4):4883–4891. doi: 10.1002/jcb.27762. [DOI] [PubMed] [Google Scholar]
- 56.Khan M. S., Khan A., Ahmad S., et al. Inhibition of JNK alleviates chronic hypoperfusion-related ischemia induces oxidative stress and brain degeneration via Nrf2/HO-1 and NF-kappaB signaling. Oxidative Medicine and Cellular Longevity. 2020;2020:18. doi: 10.1155/2020/5291852.5291852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang K., Chen Z., Huang J., et al. Naringenin prevents ischaemic stroke damage via anti-apoptotic and anti-oxidant effects. Clinical and Experimental Pharmacology & Physiology. 2017;44(8):862–871. doi: 10.1111/1440-1681.12775. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L., Zhang X., Zhang C., et al. Nobiletin promotes antioxidant and anti-inflammatory responses and elicits protection against ischemic stroke in vivo. Brain Research. 2016;1636:130–141. doi: 10.1016/j.brainres.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 59.Guo H., Kong S., Chen W., et al. Apigenin mediated protection of OGD-evoked neuron-like injury in differentiated PC12 cells. Neurochemical Research. 2014;39(11):2197–2210. doi: 10.1007/s11064-014-1421-0. [DOI] [PubMed] [Google Scholar]
- 60.Ling C., Lei C., Zou M., et al. Neuroprotective effect of apigenin against cerebral ischemia/reperfusion injury. Journal of International Medical Research. 2020;48(9, article 300060520945859) doi: 10.1177/0300060520945859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang J., Dai J., Cui H. Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomedicine & Pharmacotherapy. 2018;99:583–590. doi: 10.1016/j.biopha.2018.01.067. [DOI] [PubMed] [Google Scholar]
- 62.Wang C. P., Shi Y. W., Tang M., et al. Isoquercetin ameliorates cerebral impairment in focal ischemia through anti-oxidative, anti-inflammatory, and anti-apoptotic effects in primary culture of rat hippocampal neurons and hippocampal CA1 region of rats. Molecular Neurobiology. 2017;54(3):2126–2142. doi: 10.1007/s12035-016-9806-5. [DOI] [PubMed] [Google Scholar]
- 63.Chen M., Dai L. H., Fei A., Pan S. M., Wang H. R. Isoquercetin activates the ERK1/2-Nrf2 pathway and protects against cerebral ischemia-reperfusion injury in vivo and in vitro. Experimental and Therapeutic Medicine. 2017;13(4):1353–1359. doi: 10.3892/etm.2017.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W., Chen Z., Yan M., He P., Chen Z., Dai H. The protective role of isorhamnetin on human brain microvascular endothelial cells from cytotoxicity induced by methylglyoxal and oxygen-glucose deprivation. Journal of Neurochemistry. 2016;136(3):651–659. doi: 10.1111/jnc.13436. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y., Zhang L., Liang J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. Journal of the Neurological Sciences. 2015;351(1-2):88–92. doi: 10.1016/j.jns.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 66.Guo M., Lu H., Qin J., et al. Biochanin A provides neuroprotection against cerebral ischemia/reperfusion injury by Nrf2-mediated inhibition of oxidative stress and inflammation signaling pathway in rats. Medical Science Monitor. 2019;25:8975–8983. doi: 10.12659/MSM.918665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khamchai S., Chumboatong W., Hata J., Tocharus C., Suksamrarn A., Tocharus J. Morin protects the blood-brain barrier integrity against cerebral ischemia reperfusion through anti-inflammatory actions in rats. Scientific reports. 2020;10(1, article 13379) doi: 10.1038/s41598-020-70214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Li S., Li D. Breviscapine alleviates cognitive impairments induced by transient cerebral ischemia/reperfusion through its anti-inflammatory and anti-oxidant properties in a rat model. ACS Chemical Neuroscience. 2020;11(24):4489–4498. doi: 10.1021/acschemneuro.0c00697. [DOI] [PubMed] [Google Scholar]
- 69.An P., Wu T., Yu H., Fang K., Ren Z., Tang M. Hispidulin protects against focal cerebral ischemia reperfusion injury in rats. Journal of Molecular Neuroscience. 2018;65(2):203–212. doi: 10.1007/s12031-018-1086-2. [DOI] [PubMed] [Google Scholar]
- 70.Wu S., Yue Y., Peng A., et al. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food & Function. 2016;7(6):2624–2634. doi: 10.1039/C6FO00419A. [DOI] [PubMed] [Google Scholar]
- 71.Chao M., Gao C., Huang Y. Xanthoangelol alleviates cerebral ischemia reperfusion injury in rats. The Anatomical Record. 2021;304(3):602–612. doi: 10.1002/ar.24481. [DOI] [PubMed] [Google Scholar]
- 72.Wang J., Mao J., Wang R., Li S., Wu B., Yuan Y. Kaempferol protects against cerebral ischemia reperfusion injury through intervening oxidative and inflammatory stress induced apoptosis. Frontiers in pharmacology. 2020;11:p. 424. doi: 10.3389/fphar.2020.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong H., Ming S., Fang J., Li Y., Liu L. Icariin ameliorates angiotensin II-induced cerebrovascular remodeling by inhibiting Nox2-containing NADPH oxidase activation. Human Cell. 2019;32(1):22–30. doi: 10.1007/s13577-018-0220-3. [DOI] [PubMed] [Google Scholar]
- 74.Feng L., Gao J., Liu Y., Shi J., Gong Q. Icariside II alleviates oxygen-glucose deprivation and reoxygenation-induced PC12 cell oxidative injury by activating Nrf2/SIRT3 signaling pathway. Biomedicine & Pharmacotherapy. 2018;103:9–17. doi: 10.1016/j.biopha.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Oh T. W., Do H. J., Jeon J. H., Kim K. Quercitrin inhibits platelet activation in arterial thrombosis. Phytomedicine. 2021;80, article 153363 doi: 10.1016/j.phymed.2020.153363. [DOI] [PubMed] [Google Scholar]
- 76.Zhao Y., Xu J. Sanggenon C ameliorates cerebral ischemia-reperfusion injury by inhibiting inflammation and oxidative stress through regulating RhoA-ROCK signaling. Inflammation. 2020;43(4):1476–1487. doi: 10.1007/s10753-020-01225-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao Y., Mao X., Sun C., et al. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Research Bulletin. 2011;85(6):396–402. doi: 10.1016/j.brainresbull.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Shah Z. A., Li R. C., Ahmad A. S., et al. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO-1 pathway. Journal of Cerebral Blood Flow and Metabolism. 2010;30(12):1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leonardo C. C., Agrawal M., Singh N., Moore J. R., Biswal S., Dore S. Oral administration of the flavanol (-)-epicatechin bolsters endogenous protection against focal ischemia through the Nrf2 cytoprotective pathway. The European Journal of Neuroscience. 2013;38(11):3659–3668. doi: 10.1111/ejn.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J., Zhang Z., Lv L., Qiao H., Chen X., Zou C. (-)-Epigallocatechin gallate inhibits asymmetric dimethylarginine-induced injury in human brain microvascular endothelial cells. Neurochemical Research. 2016;41(8):1868–1876. doi: 10.1007/s11064-016-1898-9. [DOI] [PubMed] [Google Scholar]
- 81.Fu Y., Koo M. W. EGCG protects HT-22 cells against glutamate-induced oxidative stress. Neurotoxicity Research. 2006;10(1):23–29. doi: 10.1007/BF03033331. [DOI] [PubMed] [Google Scholar]
- 82.Shah F. A., Park D. J., Koh P. O. Identification of proteins differentially expressed by quercetin treatment in a middle cerebral artery occlusion model: a proteomics approach. Neurochemical Research. 2018;43(8):1608–1623. doi: 10.1007/s11064-018-2576-x. [DOI] [PubMed] [Google Scholar]
- 83.Chen B. H., Park J. H., Ahn J. H., et al. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regeneration Research. 2017;12(2):220–227. doi: 10.4103/1673-5374.200805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang H. C., Yang Y. R., Wang P. S., Wang R. Y. Quercetin enhances exercise-mediated neuroprotective effects in brain ischemic rats. Medicine and Science in Sports and Exercise. 2014;46(10):1908–1916. doi: 10.1249/MSS.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 85.Ahmad A., Khan M. M., Hoda M. N., et al. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochemical Research. 2011;36(8):1360–1371. doi: 10.1007/s11064-011-0458-6. [DOI] [PubMed] [Google Scholar]
- 86.Xu Z., Liu W., Huang H. Astragaloside IV alleviates cerebral ischemia-reperfusion injury by activating the Janus kinase 2 and signal transducer and activator of transcription 3 signaling pathway. Pharmacology. 2020;105(3-4):181–189. doi: 10.1159/000503361. [DOI] [PubMed] [Google Scholar]
- 87.Gu D. M., Lu P. H., Zhang K., et al. EGFR mediates astragaloside IV-induced Nrf2 activation to protect cortical neurons against in vitro ischemia/reperfusion damages. Biochemical and Biophysical Research Communications. 2015;457(3):391–397. doi: 10.1016/j.bbrc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Aras A. B., Guven M., Akman T., et al. Genistein exerts neuroprotective effect on focal cerebral ischemia injury in rats. Inflammation. 2015;38(3):1311–1321. doi: 10.1007/s10753-014-0102-0. [DOI] [PubMed] [Google Scholar]
- 89.Dai Y., Zhang H., Zhang J., Yan M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-kappaB pathway. Chemico-Biological Interactions. 2018;284:32–40. doi: 10.1016/j.cbi.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 90.Zhao J. J., Song J. Q., Pan S. Y., Wang K. Treatment with isorhamnetin protects the brain against ischemic injury in mice. Neurochemical Research. 2016;41(8):1939–1948. doi: 10.1007/s11064-016-1904-2. [DOI] [PubMed] [Google Scholar]
- 91.Sun L., Xu P., Fu T., et al. Myricetin against ischemic cerebral injury in rat middle cerebral artery occlusion model. Molecular Medicine Reports. 2018;17(2):3274–3280. doi: 10.3892/mmr.2017.8212. [DOI] [PubMed] [Google Scholar]
- 92.Yao Y., Chen L., Xiao J., et al. Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. International Journal of Molecular Sciences. 2014;15(11):20913–20926. doi: 10.3390/ijms151120913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shooshtari M. K., Sarkaki A., Mansouri S. M., et al. Protective effects of chrysin against memory impairment, cerebral hyperemia and oxidative stress after cerebral hypoperfusion and reperfusion in rats. Metabolic Brain Disease. 2020;35(2):401–412. doi: 10.1007/s11011-019-00527-9. [DOI] [PubMed] [Google Scholar]
- 94.Jiao Y., Cao Y., Lu X., et al. Xanthohumol protects neuron from cerebral ischemia injury in experimental stroke. Molecular Biology Reports. 2020;47(4):2417–2425. doi: 10.1007/s11033-019-05128-4. [DOI] [PubMed] [Google Scholar]
- 95.Duan W., Wang L., Lv J., et al. Metabolomics study on the effects of salvianolic acid B and borneol for treating cerebral ischemia in rats by ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry. Rejuvenation Research. 2019;22(4):313–324. doi: 10.1089/rej.2018.2099. [DOI] [PubMed] [Google Scholar]
- 96.Fan Y., Luo Q., Wei J., et al. Mechanism of salvianolic acid B neuroprotection against ischemia/reperfusion induced cerebral injury. Brain Research. 2018;1679:125–133. doi: 10.1016/j.brainres.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 97.Liu D., Wang H., Zhang Y., Zhang Z. Protective effects of chlorogenic acid on cerebral ischemia/reperfusion injury rats by regulating oxidative stress-related Nrf2 pathway. Drug Design, Development and Therapy. 2020;14:51–60. doi: 10.2147/DDDT.S228751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Kumar N., Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnology Reports. 2014;4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mancuso C., Santangelo R. Ferulic acid: pharmacological and toxicological aspects. Food and Chemical Toxicology. 2014;65:185–195. doi: 10.1016/j.fct.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 100.Cheng C. Y., Su S. Y., Tang N. Y., Ho T. Y., Chiang S. Y., Hsieh C. L. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Research. 2008;1209:136–150. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 101.Sung J. H., Gim S. A., Koh P. O. Ferulic acid attenuates the cerebral ischemic injury-induced decrease in peroxiredoxin-2 and thioredoxin expression. Neuroscience Letters. 2014;566:88–92. doi: 10.1016/j.neulet.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 102.Sun J., Li Y. Z., Ding Y. H., et al. Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Research. 2014;1589:126–139. doi: 10.1016/j.brainres.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 103.Nabavi S. F., Habtemariam S., Di Lorenzo A., et al. Post-stroke depression modulation and in vivo antioxidant activity of gallic acid and its synthetic derivatives in a murine model system. Nutrients. 2016;8(5) doi: 10.3390/nu8050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mirshekari Jahangiri H., Sarkaki A., Farbood Y., Dianat M., Goudarzi G. Gallic acid affects blood-brain barrier permeability, behaviors, hippocampus local EEG, and brain oxidative stress in ischemic rats exposed to dusty particulate matter. Environmental Science and Pollution Research International. 2020;27(5):5281–5292. doi: 10.1007/s11356-019-07076-9. [DOI] [PubMed] [Google Scholar]
- 105.Min L. Q., Dang L. Y., Ma W. Y. Clinical study on effect and therapeutical mechanism of composite Salvia injection on acute cerebral infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22(5):353–355. [PubMed] [Google Scholar]
- 106.Miao M., Xiang L. Pharmacological action and potential targets of chlorogenic acid. Advances in Pharmacology. 2020;87:71–88. doi: 10.1016/bs.apha.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Grabska-Kobylecka I., Kaczmarek-Bak J., Figlus M., et al. The presence of caffeic acid in cerebrospinal fluid: evidence that dietary polyphenols can cross the blood-brain barrier in humans. Nutrients. 2020;12(5) doi: 10.3390/nu12051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang G., Shi B., Luo W., Yang J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behavioral and Brain Functions. 2015;11:p. 18. doi: 10.1186/s12993-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou Y., Fang S. H., Ye Y. L., et al. Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats. Acta Pharmacologica Sinica. 2006;27(9):1103–1110. doi: 10.1111/j.1745-7254.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 110.Alfei S., Turrini F., Catena S., et al. Ellagic acid a multi-target bioactive compound for drug discovery in CNS? A narrative review. European journal of medicinal chemistry. 2019;183, article 111724 doi: 10.1016/j.ejmech.2019.111724. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y., Wu Y., Liang C., Tan R., Tan L., Tan R. Pharmacodynamic effect of ellagic acid on ameliorating cerebral ischemia/reperfusion injury. Pharmacology. 2019;104(5-6):320–331. doi: 10.1159/000502401. [DOI] [PubMed] [Google Scholar]
- 112.Cui H. Y., Zhang X. J., Yang Y., et al. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regeneration Research. 2018;13(12):2119–2128. doi: 10.4103/1673-5374.241463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He Y., Yue Y., Zheng X., Zhang K., Chen S., Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20(5):9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giordano A., Tommonaro G. Curcumin and cancer. Nutrients. 2019;11(10) doi: 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li H., Sureda A., Devkota H. P., et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnology advances. 2020;38, article 107343 doi: 10.1016/j.biotechadv.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 116.Thiyagarajan M., Sharma S. S. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sciences. 2004;74(8):969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 117.Zhao J., Yu S., Zheng W., et al. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochemical Research. 2010;35(3):374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- 118.Pu Y., Zhang H., Wang P., et al. Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the AMPK/uncoupling protein 2 pathway. Cellular Physiology and Biochemistry. 2013;32(5):1167–1177. doi: 10.1159/000354516. [DOI] [PubMed] [Google Scholar]
- 119.Lan C., Chen X., Zhang Y., et al. Curcumin prevents strokes in stroke-prone spontaneously hypertensive rats by improving vascular endothelial function. BMC cardiovascular disorders. 2018;18(1):1–10. doi: 10.1186/s12872-018-0768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y., Li J., Li S., et al. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicology and Applied Pharmacology. 2015;286(1):53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 121.Wu J., Li Q., Wang X., et al. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One. 2013;8(3, article e59843) doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jia G., Tan B., Ma J., Zhang L., Jin X., Li C. Prdx6 upregulation by curcumin attenuates ischemic oxidative damage via SP1 in rats after stroke. BioMed Research International. 2017;2017 doi: 10.1155/2017/6597401.6597401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu L., Jiang C., Kang Y., Dai Y., Fang W., Huang P. Curcumin exerts protective effects against hypoxiareoxygenation injury via the enhancement of apurinic/apyrimidinic endonuclease 1 in SHSY5Y cells: involvement of the PI3K/AKT pathway. International Journal of Molecular Medicine. 2020;45(4):993–1004. doi: 10.3892/ijmm.2020.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mondal N. K., Behera J., Kelly K. E., George A. K., Tyagi P. K., Tyagi N. Tetrahydrocurcumin epigenetically mitigates mitochondrial dysfunction in brain vasculature during ischemic stroke. Neurochemistry International. 2019;122:120–138. doi: 10.1016/j.neuint.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin B., Yu H., Lin Y., Cai C., Lu H., Zhu X. Suppression of GRASP65 phosphorylation by tetrahydrocurcumin protects against cerebral ischemia/reperfusion injury via ERK signaling. Molecular Medicine Reports. 2016;14(5):4775–4780. doi: 10.3892/mmr.2016.5816. [DOI] [PubMed] [Google Scholar]
- 126.Wicha P., Tocharus J., Janyou A., et al. Hexahydrocurcumin protects against cerebral ischemia/reperfusion injury, attenuates inflammation, and improves antioxidant defenses in a rat stroke model. PLoS One. 2017;12(12, article e0189211) doi: 10.1371/journal.pone.0189211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ahmad N., Umar S., Ashafaq M., et al. A comparative study of PNIPAM nanoparticles of curcumin, demethoxycurcumin, and bisdemethoxycurcumin and their effects on oxidative stress markers in experimental stroke. Protoplasma. 2013;250(6):1327–1338. doi: 10.1007/s00709-013-0516-9. [DOI] [PubMed] [Google Scholar]
- 128.Fodor K., Tit D. M., Pasca B., et al. Long-term resveratrol supplementation as a secondary prophylaxis for stroke. Oxidative Medicine and Cellular Longevity. 2018;2018:10. doi: 10.1155/2018/4147320.4147320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sinha K., Chaudhary G., Gupta Y. K. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sciences. 2002;71(6):655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- 130.de Almeida L. M., Leite M. C., Thomazi A. P., et al. Resveratrol protects against oxidative injury induced by H2O2 in acute hippocampal slice preparations from Wistar rats. Archives of Biochemistry and Biophysics. 2008;480(1):27–32. doi: 10.1016/j.abb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 131.Agrawal M., Kumar V., Kashyap M. P., Khanna V. K., Randhawa G. S., Pant A. B. Ischemic insult induced apoptotic changes in PC12 cells: protection by _trans_ resveratrol. European Journal of Pharmacology. 2011;666(1-3):5–11. doi: 10.1016/j.ejphar.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 132.Orsu P., Murthy B. V., Akula A. Cerebroprotective potential of resveratrol through anti-oxidant and anti-inflammatory mechanisms in rats. Journal of Neural Transmission. 2013;120(8):1217–1223. doi: 10.1007/s00702-013-0982-4. [DOI] [PubMed] [Google Scholar]
- 133.Prabhakar O. Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 2013;386(8):705–710. doi: 10.1007/s00210-013-0871-2. [DOI] [PubMed] [Google Scholar]
- 134.Su Q., Pu H., Hu C. Neuroprotection by combination of resveratrol and enriched environment against ischemic brain injury in rats. Neurological Research. 2016;38(1):60–68. doi: 10.1080/01616412.2015.1133027. [DOI] [PubMed] [Google Scholar]
- 135.Hermann D. M., Zechariah A., Kaltwasser B., et al. Sustained neurological recovery induced by resveratrol is associated with angioneurogenesis rather than neuroprotection after focal cerebral ischemia. Neurobiology of Disease. 2015;83:16–25. doi: 10.1016/j.nbd.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 136.Ham P. B., 3rd, Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Progress in Neurobiology. 2017;157:92–116. doi: 10.1016/j.pneurobio.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khoury N., Xu J., Stegelmann S. D., et al. Resveratrol preconditioning induces genomic and metabolic adaptations within the long-term window of cerebral ischemic tolerance leading to bioenergetic efficiency. Molecular Neurobiology. 2019;56(6):4549–4565. doi: 10.1007/s12035-018-1380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yousuf S., Atif F., Ahmad M., et al. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Research. 2009;1250:242–253. doi: 10.1016/j.brainres.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 139.Jiao F., Gong Z. The beneficial roles of SIRT1 in neuroinflammation-related diseases. Oxidative Medicine and Cellular Longevity. 2020;2020:19. doi: 10.1155/2020/6782872.6782872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Man A. W. C., Li H., Xia N. The role of sirtuin1 in regulating endothelial function, arterial remodeling and vascular aging. Frontiers in physiology. 2019;10, article 1173 doi: 10.3389/fphys.2019.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clark D., Tuor U. I., Thompson R., et al. Protection against recurrent stroke with resveratrol: endothelial protection. PLoS One. 2012;7(10, article e47792) doi: 10.1371/journal.pone.0047792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shin J. A., Lee K. E., Kim H. S., Park E. M. Acute resveratrol treatment modulates multiple signaling pathways in the ischemic brain. Neurochemical Research. 2012;37(12):2686–2696. doi: 10.1007/s11064-012-0858-2. [DOI] [PubMed] [Google Scholar]
- 143.Yang J., Huang J., Shen C., et al. Resveratrol treatment in different time-attenuated neuronal apoptosis after oxygen and glucose deprivation/reoxygenation via enhancing the activation of Nrf-2 signaling pathway in vitro. Cell Transplantation. 2018;27(12):1789–1797. doi: 10.1177/0963689718780930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shen C., Cheng W., Yu P., et al. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Molecular Medicine Reports. 2016;14(4):3646–3654. doi: 10.3892/mmr.2016.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ren J., Fan C., Chen N., Huang J., Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochemical Research. 2011;36(12):2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 146.Huang S. Y., Tai S. H., Chang C. C., Tu Y. F., Chang C. H., Lee E. J. Magnolol protects against ischemic-reperfusion brain damage following oxygen-glucose deprivation and transient focal cerebral ischemia. International Journal of Molecular Medicine. 2018;41(4):2252–2262. doi: 10.3892/ijmm.2018.3387. [DOI] [PubMed] [Google Scholar]
- 147.Eggler A. L., Liu G., Pezzuto J. M., van Breemen R. B., Mesecar A. D. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(29):10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rachakonda G., Xiong Y., Sekhar K. R., Stamer S. L., Liebler D. C., Freeman M. L. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chemical Research in Toxicology. 2008;21(3):705–710. doi: 10.1021/tx700302s. [DOI] [PubMed] [Google Scholar]
- 149.Mills E. L., Ryan D. G., Prag H. A., et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Q., Lenardo M. J., Baltimore D. 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168(1-2):37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morgan M. J., Liu Z. G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Research. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baker R. G., Hayden M. S., Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metabolism. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aggarwal V., Tuli H. S., Varol A., et al. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;9(11) doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shirley R., Ord E. N., Work L. M. Oxidative stress and the use of antioxidants in stroke. Antioxidants. 2014;3(3):472–501. doi: 10.3390/antiox3030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hankey G. J. Stroke. Lancet. 2017;389(10069):641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 156.Zhou Y., Men L., Pi Z., et al. Fecal metabolomics of type 2 diabetic rats and treatment with Gardenia jasminoides Ellis based on mass spectrometry technique. Journal of Agricultural and Food Chemistry. 2018;66(6):1591–1599. doi: 10.1021/acs.jafc.7b06082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this review article.


