Figure 1.
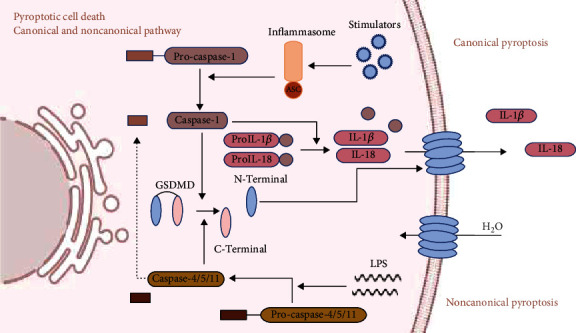
Canonical and noncanonical pyroptosis pathways. The canonical pyroptosis pathway requires the involvement of inflammasomes. Inflammasomes are multiprotein complexes assembled in response to external stimuli, such as hypoxia, injury, toxins, and pathogens. A typical inflammasome contains a pattern recognition receptor and a downstream adaptor such as apoptosis-associated speck-like (ASC) protein. Four main inflammasomes have been identified: NLR members NOD-, LRR-, and pyrin domain containing 1 (NLRP1); pyrin domain containing 3 (NLRP3); NOD-, LRR-, and caspase recruitment domain containing 4 (NLRC4); and absent in melanoma 2 (AIM2), a sensor for nucleic acids. Different inflammasomes receive different stimulatory signals. Activation of inflammasomes promotes maturation of procaspase-1 into caspase-1, which further cleaves immature prointerleukin- (pro-IL-) 1β and pro-IL-18 into mature IL-1β and IL-18. Caspase-1 cleaves GSDMD into N- and C-terminal components. The N-terminal domains bind to cell membranes to form oligomeric pores, causing lytic cell death. In the noncanonical pyroptosis pathway, caspase-11 and caspase-4/5 are involved and inflammasomes are not needed. The stimulatory signals mainly come from Gram-negative bacterial lipopolysaccharide (LPS). LPS activates caspase-11 (caspase-4/5 in humans) directly and mature caspase-4/5/11 cleave GSDMD to trigger pyroptosis. Caspase-4/5/11 do not have the function to process pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18. However, inflammatory cytokines released in the noncanonical pyroptosis pathway have been observed, which indicates the probable interaction between the two pathways.
