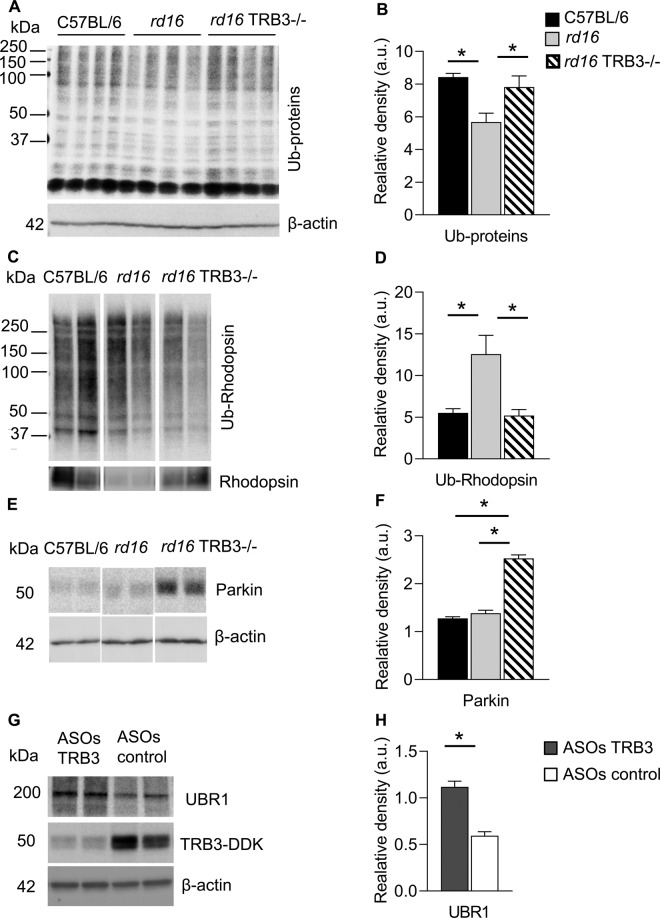
Fig. 5. TRB3 ablation in rd16 retinas led to the restoration of the ubiquitinated protein level and an increase in E3 ligase expression.
A Images of western blot membrane probed with anti-Ub antibody to detect level of total protein ubiquitination. B Calculation of the band density measured for western blot (A). Significant reduction in the total Ub-protein levels was detected in rd16 retinas (n = 8). C, D The Ub-rhodopsin level was detected by performing the IP reaction using protein extracts from C56BL/6, rd16, and rd16 TRB3−/− mice. C Images of western blot probed with anti-Ub and Rhodopsin antibodies. D Levels of Ub-rhodopsin from IP reactions were normalized through total rhodopsin protein. Measurement indicated that rhodopsin protein was highly ubiquitinated in the rd16 retinas. E, F Parkin 1 ligase expression in the retinas of C56BL/6, rd16, and rd16 TRB3−/− mice and MIO-M1 Müller cells (n = 4). E Images of western blot obtained with retinal protein extracts from P15 C56BL/6, rd16, and rd16 TRB3−/− mice probed with anti-Ubr1, Parkin 1, and β-actin antibodies. F TRB3 ablation in rd16 resulted in increase in Parkin 1 protein compared to both the C57BL/6 and rd16 mice. G, H Sequential transfection of Müller cells with plasmid expressing TRB3 cDNA and ASO-TRB3 (n = 4). G Images of western blots running with protein extracts probed with anti-UBR1, TRB3, and β-actin antibodies. H Quantitation of UBR1 expression in cells co-transfected with ASO-control and ASO-TRB3. A significant decrease in UBR1 level was observed in cells transfected with ASO-TRB3. Data are shown as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.