Figure 7.
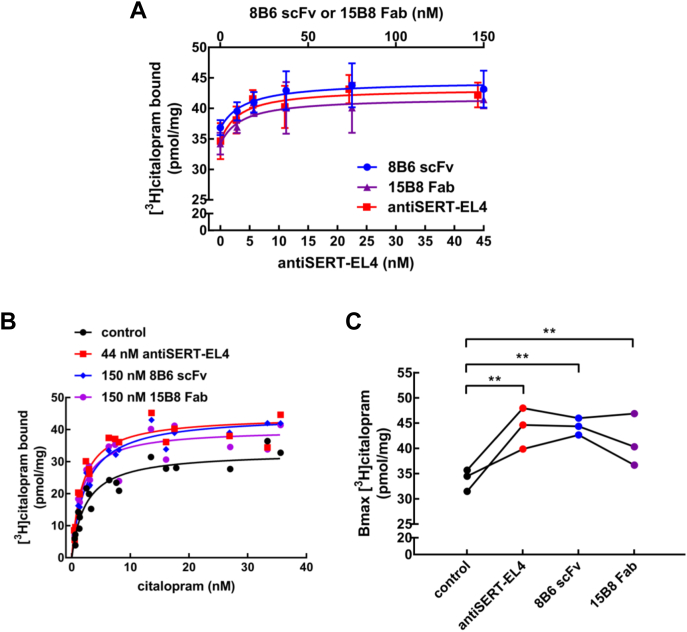
Binding of [3H]citalopram to SERT in the presence of 8B6 scFv, 15B8 Fab, or of the antiSERT-EL4 antibody.A, concentration–response curve for 8B6 scFv, 15B8 Fab, and antiSERT-EL4 antibody in enhancing binding of [3H]citalopram to SERT. The experiments were carried out as outlined for [3H]imipramine binding in Figure 6C. The concentration of [3H]citalopram was 10 nM. The concentrations were 9.5, 19, 37.5, 75, and 150 nM for both 8B6 scFv and 15B8 Fab (denoted on the top x-axis), whereas the antiSERT-EL4 antibody concentrations were 2.7, 5.5, 11, 22, and 44 nM (denoted on the bottom x-axis). Data are means ± SD from three independent experiments carried out in duplicate. The lines were drawn by fitting the data to the equation for a rectangular hyperbola + basal binding in the absence of Fab/antibody. EC50 values were 14.7 ± 2.3 nM, 11.2 ± 2.5 nM, and 5.0 ± 2.7 nM for 8B6 scFv, 15B8 Fab, and antiSERT-EL4, respectively. B, saturation of [3H]citalopram binding to SERT in the absence and presence of 8B6 scFv (150 nM), 15B8 Fab (150 nM), or antiSERT-EL4 (44 nM). The reaction was done as in panel A with [3H]citalopram concentrations ranging from 0.4 to 36 nM. Shown are the pooled data from three experiments carried out in duplicate. The lines were drawn by fitting the data to a rectangular hyperbola. C, the spaghetti plot depicts the change in Bmax of [3H]citalopram binding in the presence of 8B6 scFv, 15B8 Fab, or antiSERT-EL4 antibody in three independent experiments carried out in duplicate (statistical significance was tested by repeated-measures ANOVA, followed by Holm–Sidak's multiple comparisons; Bmax in the presence of 8B6 scFv, 15B8 Fab, or of antiSERT-EL4 antibody differed from control Bmax, p < 0.005).